NEET-XI-Chemistry
05: Organic Chemistry: Some Basic Principles and Techniques
- #1-iCH2=C=O, () CH3CH=CH2, () (CH3)2CO, () CH2=CHCN, () C6H6
() CH3CH=CH2, () (CH3)2CO, () CH2=CHCN, () C6H6
() CH3CH=CH2, () (CH3)2CO, () CH2=CHCN, () C6H6Ans :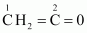
C-1 is sp2 hybridised.
C-2 is sp hybridised.
()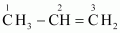
C-1 is sp3 hybridised.
C-2 is sp2 hybridised.
C-3 is sp2 hybridised.
()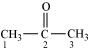
C-1 and C-3 are sp3 hybridised.
C-2 is sp2 hybridised.
()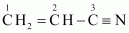
C-1 is sp2 hybridised.
C-2 is sp2 hybridised.
C-3 is sp hybridised.
() C6H6
All the 6 carbon atoms in benzene are sp2 hybridised.
()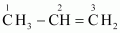
C-1 is sp3 hybridised.
C-2 is sp2 hybridised.
C-3 is sp2 hybridised.
()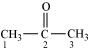
C-1 and C-3 are sp3 hybridised.
C-2 is sp2 hybridised.
()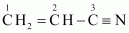
C-1 is sp2 hybridised.
C-2 is sp2 hybridised.
C-3 is sp hybridised.
() C6H6
All the 6 carbon atoms in benzene are sp2 hybridised.
()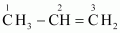
C-1 is sp3 hybridised.
C-2 is sp2 hybridised.
C-3 is sp2 hybridised.
()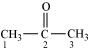
C-1 and C-3 are sp3 hybridised.
C-2 is sp2 hybridised.
()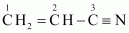
C-1 is sp2 hybridised.
C-2 is sp2 hybridised.
C-3 is sp hybridised.
() C6H6
All the 6 carbon atoms in benzene are sp2 hybridised.
- #1-iiCH3CH=CH2,Ans :
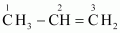
C-1 is sp3 hybridised.
C-2 is sp2 hybridised.
C-3 is sp2 hybridised.
- #1-iii(CH3)2CO,Ans :
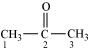
C-1 and C-3 are sp3 hybridised.
C-2 is sp2 hybridised.
- #1-ivCH2=CHCN,Ans :
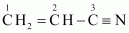
C-1 is sp2 hybridised.
C-2 is sp2 hybridised.
C-3 is sp hybridised.
- #1-vC6H6Ans : C6H6
All the 6 carbon atoms in benzene are sp2 hybridised.