NEET-XI-Chemistry
05: Organic Chemistry: Some Basic Principles and Techniques
- #2Indicate the σ and ``\pi`` bonds in the following molecules:
() C6H6, () C6H12, () CH2Cl2, () CH2 = C = CH2, () CH3NO2, () HCONHCH3
() C6H6, () C6H12, () CH2Cl2, () CH2 = C = CH2, () CH3NO2, () HCONHCH3Ans : null () C6H6
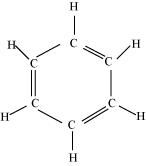
There are six C-C sigma (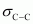 ) bonds, six C-H sigma (
) bonds, six C-H sigma (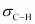 ) bonds, and three C=C pi (
) bonds, and three C=C pi (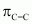 ) resonating bonds in the given compound.
) resonating bonds in the given compound.
() C6H12
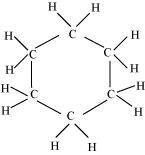
There are six C-C sigma (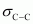 ) bonds and twelve C-H sigma (
) bonds and twelve C-H sigma (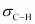 ) bonds in the given compound.
) bonds in the given compound.
() CH2Cl2
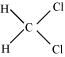
There two C-H sigma (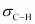 ) bonds and two C-Cl sigma (
) bonds and two C-Cl sigma (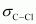 ) bonds in the given compound.
) bonds in the given compound.
() CH2 = C = CH2
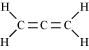
There are two C-C sigma (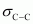 ) bonds, four C-H sigma (
) bonds, four C-H sigma (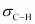 ) bonds, and two C=C pi (
) bonds, and two C=C pi (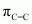 ) bonds in the given compound.
) bonds in the given compound.
() CH3NO2
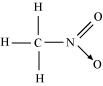
There are three C-H sigma (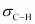 ) bonds, one C-N sigma (
) bonds, one C-N sigma (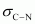 ) bond, one N-O sigma (
) bond, one N-O sigma (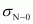 ) bond, and one N=O pi (
) bond, and one N=O pi (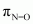 ) bond in the given compound.
) bond in the given compound.
() HCONHCH3
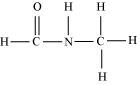
There are two C-N sigma (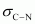 ) bonds, four C-H sigma (
) bonds, four C-H sigma (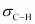 ) bonds, one N-H sigma bond, and one C=O pi (
) bonds, one N-H sigma bond, and one C=O pi (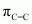 ) bond in the given compound.
) bond in the given compound.
() C6H6

There are six C-C sigma (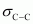 ) bonds, six C-H sigma (
) bonds, six C-H sigma (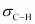 ) bonds, and three C=C pi (
) bonds, and three C=C pi (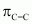 ) resonating bonds in the given compound.
) resonating bonds in the given compound.
() C6H12

There are six C-C sigma (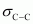 ) bonds and twelve C-H sigma (
) bonds and twelve C-H sigma (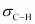 ) bonds in the given compound.
) bonds in the given compound.
() CH2Cl2

There two C-H sigma (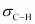 ) bonds and two C-Cl sigma (
) bonds and two C-Cl sigma (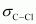 ) bonds in the given compound.
) bonds in the given compound.
() CH2 = C = CH2
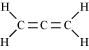
There are two C-C sigma (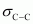 ) bonds, four C-H sigma (
) bonds, four C-H sigma (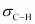 ) bonds, and two C=C pi (
) bonds, and two C=C pi (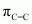 ) bonds in the given compound.
) bonds in the given compound.
() CH3NO2

There are three C-H sigma (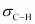 ) bonds, one C-N sigma (
) bonds, one C-N sigma (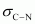 ) bond, one N-O sigma (
) bond, one N-O sigma (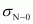 ) bond, and one N=O pi (
) bond, and one N=O pi (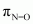 ) bond in the given compound.
) bond in the given compound.
() HCONHCH3

There are two C-N sigma (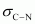 ) bonds, four C-H sigma (
) bonds, four C-H sigma (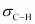 ) bonds, one N-H sigma bond, and one C=O pi (
) bonds, one N-H sigma bond, and one C=O pi (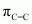 ) bond in the given compound.
) bond in the given compound.
- #2-iC6H6,Ans : C6H6

There are six C-C sigma (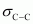 ) bonds, six C-H sigma (
) bonds, six C-H sigma (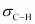 ) bonds, and three C=C pi (
) bonds, and three C=C pi (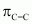 ) resonating bonds in the given compound.
) resonating bonds in the given compound.
- #2-iiC6H12,Ans : C6H12

There are six C-C sigma (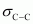 ) bonds and twelve C-H sigma (
) bonds and twelve C-H sigma (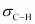 ) bonds in the given compound.
) bonds in the given compound.
- #2-iiiCH2Cl2,Ans : CH2Cl2

There two C-H sigma (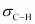 ) bonds and two C-Cl sigma (
) bonds and two C-Cl sigma (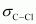 ) bonds in the given compound.
) bonds in the given compound.
- #2-ivCH2 = C = CH2,Ans : CH2 = C = CH2
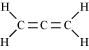
There are two C-C sigma (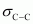 ) bonds, four C-H sigma (
) bonds, four C-H sigma (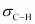 ) bonds, and two C=C pi (
) bonds, and two C=C pi (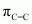 ) bonds in the given compound.
) bonds in the given compound.
- #2-vCH3NO2,Ans : CH3NO2

There are three C-H sigma (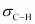 ) bonds, one C-N sigma (
) bonds, one C-N sigma (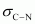 ) bond, one N-O sigma (
) bond, one N-O sigma (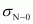 ) bond, and one N=O pi (
) bond, and one N=O pi (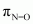 ) bond in the given compound.
) bond in the given compound.
- #2-viHCONHCH3Ans : HCONHCH3

There are two C-N sigma (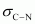 ) bonds, four C-H sigma (
) bonds, four C-H sigma (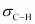 ) bonds, one N-H sigma bond, and one C=O pi (
) bonds, one N-H sigma bond, and one C=O pi (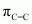 ) bond in the given compound.
) bond in the given compound.