NEET-XI-Chemistry
04: The p-Block Elements
- #11What is the state of hybridisation of carbon in (a)
.gif) (b) diamond
(b) diamond
(c) graphite?
Ans : The state of hybridisation of carbon in:
(a)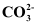
C in.gif) is sp2 hybridised and is bonded to three oxygen atoms.
is sp2 hybridised and is bonded to three oxygen atoms.
(b) Diamond
Each carbon in diamond is sp3 hybridised and is bound to four other carbon atoms.
(c) Graphite
Each carbon atom in graphite is sp2 hybridised and is bound to three other carbon atoms.
- #11-a
.gif) (b) diamond
(b) diamond
(c) graphite?
Ans :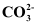
C in.gif) is sp2 hybridised and is bonded to three oxygen atoms.
is sp2 hybridised and is bonded to three oxygen atoms.
(b) Diamond
Each carbon in diamond is sp3 hybridised and is bound to four other carbon atoms.
(c) Graphite
Each carbon atom in graphite is sp2 hybridised and is bound to three other carbon atoms.