NEET-XI-Chemistry
01: Redox Reactions
- #1Assign oxidation numbers to the underlined elements in each of the following species:
(a) NaH2PO4 (b) NaHSO4 (c) H4P2O7 (d) K2MnO4
(e) CaO2 (f) NaBH4 (g) H2S2O7 (h) KAl(SO4)2.12 H2OAns : null (a)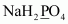
Let the oxidation number of P be x.
We know that,
Oxidation number of Na = +1
Oxidation number of H = +1
Oxidation number of O = -2
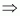
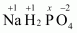
Then, we have
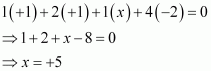
Hence, the oxidation number of P is +5.
(b)
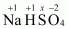
Then, we have

Hence, the oxidation number of S is + 6.
(c)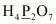
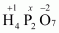
Then, we have
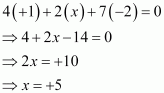
Hence, the oxidation number of P is + 5.
(d)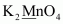
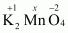
Then, we have
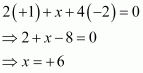
Hence, the oxidation number of Mn is + 6.
(e)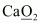
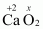
Then, we have
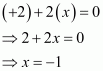
Hence, the oxidation number of O is - 1.
(f)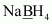
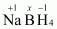
Then, we have
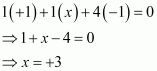
Hence, the oxidation number of B is + 3.
(g)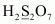
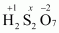
Then, we have
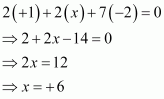
Hence, the oxidation number of S is + 6.
(h)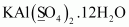
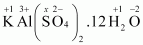
Then, we have
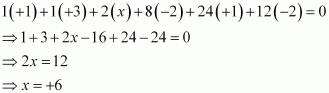
Or,
We can ignore the water molecule as it is a neutral molecule. Then, the sum of the oxidation numbers of all atoms of the water molecule may be taken as zero. Therefore, after ignoring the water molecule, we have
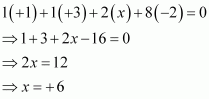
Hence, the oxidation number of S is + 6.
- #1-aNaH2PO4Ans :
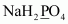
Let the oxidation number of P be x.
We know that,
Oxidation number of Na = +1
Oxidation number of H = +1
Oxidation number of O = -2
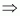
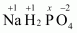
Then, we have
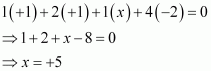
Hence, the oxidation number of P is +5.
- #1-bNaHSO4Ans :

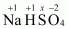
Then, we have

Hence, the oxidation number of S is + 6.
- #1-cH4P2O7Ans :
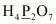
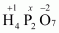
Then, we have
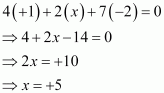
Hence, the oxidation number of P is + 5.
- #1-dK2MnO4Ans :
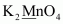
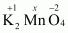
Then, we have
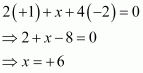
Hence, the oxidation number of Mn is + 6.
- #1-eCaO2Ans :
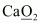
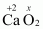
Then, we have
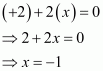
Hence, the oxidation number of O is - 1.
- #1-fNaBH4Ans :
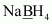
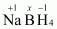
Then, we have
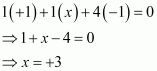
Hence, the oxidation number of B is + 3.
- #1-gH2S2O7Ans :
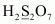
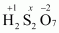
Then, we have
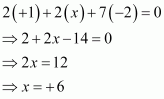
Hence, the oxidation number of S is + 6.
- #1-hKAl(SO4)2.12 H2OAns :
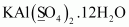
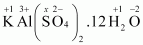
Then, we have
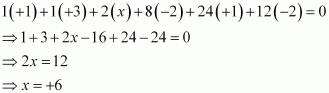
Or,
We can ignore the water molecule as it is a neutral molecule. Then, the sum of the oxidation numbers of all atoms of the water molecule may be taken as zero. Therefore, after ignoring the water molecule, we have
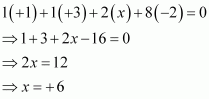
Hence, the oxidation number of S is + 6.