NEET-XI-Chemistry
01: Redox Reactions
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
No item to list.
Note: Please signup/signin free to get personalized experience.
- #Chapter 1 - Redox Reactions
- #1-aNaH2PO4Ans :
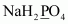
Let the oxidation number of P be x.
We know that,
Oxidation number of Na = +1
Oxidation number of H = +1
Oxidation number of O = -2
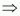
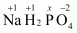
Then, we have
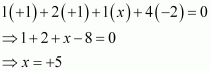
Hence, the oxidation number of P is +5.
- #1-bNaHSO4Ans :

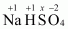
Then, we have

Hence, the oxidation number of S is + 6.
- #1-cH4P2O7Ans :
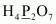
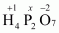
Then, we have
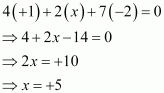
Hence, the oxidation number of P is + 5.
- #1-dK2MnO4Ans :
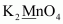
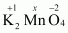
Then, we have
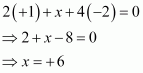
Hence, the oxidation number of Mn is + 6.
- #1-eCaO2Ans :
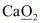
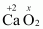
Then, we have
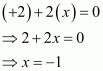
Hence, the oxidation number of O is - 1.
- #1-fNaBH4Ans :
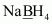
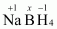
Then, we have
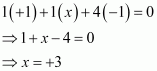
Hence, the oxidation number of B is + 3.
- #1-gH2S2O7Ans :
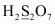
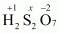
Then, we have
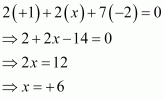
Hence, the oxidation number of S is + 6.
- #1-hKAl(SO4)2.12 H2OAns :
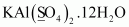
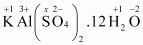
Then, we have
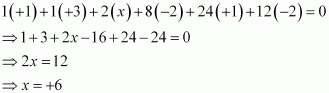
Or,
We can ignore the water molecule as it is a neutral molecule. Then, the sum of the oxidation numbers of all atoms of the water molecule may be taken as zero. Therefore, after ignoring the water molecule, we have
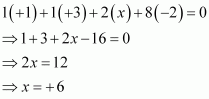
Hence, the oxidation number of S is + 6.
- Qstn #2What are the oxidation numbers of the underlined elements in each of the following and how do you rationalise your results?
- #2-aKI3 (b) H2S4O6 (c) Fe3O4 (d) CH3CH2OH (e) CH3COOHAns : KI3
In KI3, the oxidation number (O.N.) of K is +1. Hence, the average oxidation number of I is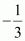 . However, O.N. cannot be fractional. Therefore, we will have to consider the structure of KI3to find the oxidation states.
. However, O.N. cannot be fractional. Therefore, we will have to consider the structure of KI3to find the oxidation states.
In a KI3molecule, an atom of iodine forms a coordinate covalent bond with an iodine molecule.
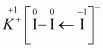
Hence, in a KI3molecule, the O.N. of the two I atoms forming the I2molecule is 0, whereas the O.N. of the I atom forming the coordinate bond is –1.
(b) H2S4O6
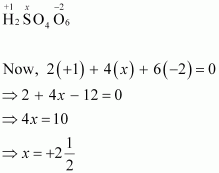
However, O.N. cannot be fractional. Hence, S must be present in different oxidation states in the molecule.
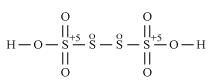
The O.N. of two of the four S atoms is +5 and the O.N. of the other two S atoms is 0.
(c)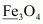
On taking the O.N. of O as –2, the O.N. of Fe is found to be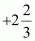 . However, O.N. cannot be fractional.
. However, O.N. cannot be fractional.
Here, one of the three Fe atoms exhibits the O.N. of +2 and the other two Fe atoms exhibit the O.N. of +3.
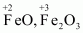
(d)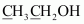
2 (x) + 6 (+1) + 1 (-2) = 0
or, 2x + 4 = 0
or, x = -2
Hence, the O.N. of C is –2.
(e)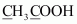
2 (x) + 4 (+1) + 2 (-2) = 0
or, 2x = 0
or, x = 0
However, 0 is average O.N. of C. The two carbon atoms present in this molecule are present in different environments. Hence, they cannot have the same oxidation number. Thus, C exhibits the oxidation states of +2 and –2 in CH3COOH.
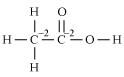
- #2-bH2S4O6Ans : H2S4O6
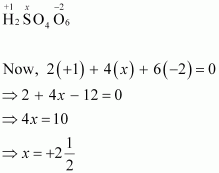
However, O.N. cannot be fractional. Hence, S must be present in different oxidation states in the molecule.
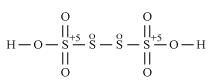
The O.N. of two of the four S atoms is +5 and the O.N. of the other two S atoms is 0.
- #2-cFe3O4Ans :
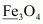
On taking the O.N. of O as –2, the O.N. of Fe is found to be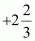 . However, O.N. cannot be fractional.
. However, O.N. cannot be fractional.
Here, one of the three Fe atoms exhibits the O.N. of +2 and the other two Fe atoms exhibit the O.N. of +3.
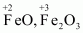
- #2-dCH3CH2OHAns :
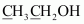
2 (x) + 6 (+1) + 1 (-2) = 0
or, 2x + 4 = 0
or, x = -2
Hence, the O.N. of C is –2.