NEET-XI-Chemistry
04: Chemical Bonding and Molecular Structure
- #3Write Lewis symbols for the following atoms and ions:
S and S2-; Al and Al3+; H and H-
Ans : (i) S and S2-
The number of valence electrons in sulphur is 6.
The Lewis dot symbol of sulphur (S) is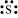 .
.
The dinegative charge infers that there will be two electrons more in addition to the six valence electrons. Hence, the Lewis dot symbol of S2- is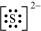 .
.
(ii) Al and Al3+
The number of valence electrons in aluminium is 3.
The Lewis dot symbol of aluminium (Al) is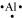 .
.
The tripositive charge on a species infers that it has donated its three electrons. Hence, the Lewis dot symbol is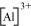 .
.
(iii) H and H-
The number of valence electrons in hydrogen is 1.
The Lewis dot symbol of hydrogen (H) is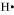 .
.
The uninegative charge infers that there will be one electron more in addition to the one valence electron. Hence, the Lewis dot symbol is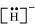 .
.