NEET-XI-Chemistry
04: Chemical Bonding and Molecular Structure
- #2Write Lewis dot symbols for atoms of the following elements: Mg, Na, B, O, N, Br.
Ans : Mg: There are two valence electrons in Mg atom. Hence, the Lewis dot symbol for Mg is: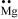
Na: There is only one valence electron in an atom of sodium. Hence, the Lewis dot structure is: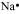
B: There are 3 valence electrons in Boron atom. Hence, the Lewis dot structure is: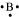
O: There are six valence electrons in an atom of oxygen. Hence, the Lewis dot structure is: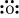
N: There are five valence electrons in an atom of nitrogen. Hence, the Lewis dot structure is: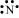
Br: There are seven valence electrons in bromine. Hence, the Lewis dot structure is: