NEET-XI-Chemistry
01: Some Basic Concepts of Chemistry
Qstn# 21-i Prvs-Qstn
- #21-i14 g 16 g
() 14 g 32 g
() 28 g 32 g
(iv) 28 g 80 g
(a) Which law of chemical combination is obeyed by the above experimental data?Give its statement.
(b) Fill in the blanks in the following conversions:
(b) 1 km = ...................... mm = ...................... pm
(b) 1 mg = ...................... kg = ...................... ng
(b) 1 mL = ...................... L = ...................... dm3
() 14 g 32 g
() 28 g 32 g
(iv) 28 g 80 g
(a) Which law of chemical combination is obeyed by the above experimental data?Give its statement.
(b) Fill in the blanks in the following conversions:
(b) 1 km = ...................... mm = ...................... pm
(b) 1 mg = ...................... kg = ...................... ng
(b) 1 mL = ...................... L = ...................... dm3
() 14 g 32 g
() 28 g 32 g
(iv) 28 g 80 g
(a) Which law of chemical combination is obeyed by the above experimental data?Give its statement.
(b) Fill in the blanks in the following conversions:
(b) 1 km = ...................... mm = ...................... pm
(b) 1 mg = ...................... kg = ...................... ng
(b) 1 mL = ...................... L = ...................... dm3Ans : 1 mole (44 g) of CO2 contains 12 g of carbon.
∴ 3.38 g of CO2 will contain carbon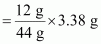
= 0.9217 g
18 g of water contains 2 g of hydrogen.
∴ 0.690 g of water will contain hydrogen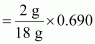
= 0.0767 g
Since carbon and hydrogen are the only constituents of the compound, the total mass of the compound is:
= 0.9217 g + 0.0767 g
= 0.9984 g
∴ Percent of C in the compound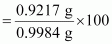
= 92.32%
Percent of H in the compound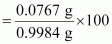
= 7.68%
Moles of carbon in the compound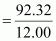
= 7.69
Moles of hydrogen in the compound =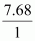
= 7.68
∴ Ratio of carbon to hydrogen in the compound = 7.69: 7.68
= 1: 1
Hence, the empirical formula of the gas is CH.
() Given,
Weight of 10.0L of the gas (at S.T.P) = 11.6 g
∴ Weight of 22.4 L of gas at STP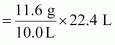
= 25.984 g
≈ 26 g
Hence, the molar mass of the gas is 26 g.
() Empirical formula mass of CH = 12 + 1 = 13 g
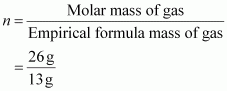
n = 2
∴ Molecular formula of gas = (CH)n
= C2H2
35:
Calcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 according to the reaction, CaCO3(s) + 2 HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
What mass of CaCO3 is required to react completely with 25 mL of 0.75 M HCl?
(a) If we fix the mass of dinitrogen at 28 g, then the masses of dioxygen that will combine with the fixed mass of dinitrogen are 32 g, 64 g, 32 g, and 80 g.
The masses of dioxygen bear a whole number ratio of 1:2:2:5. Hence, the given experimental data obeys the law of multiple proportions. The law states that if two elements combine to form more than one compound, then the masses of one element that combines with the fixed mass of another element are in the ratio of small whole numbers.
(b) null (b) 1 km = 1 km ×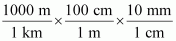
∴ 1 km = 106 mm
1 km = 1 km ×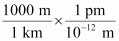
∴ 1 km = 1015 pm
Hence, 1 km = 106 mm = 1015 pm
(b) 1 mg = 1 mg ×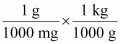
⇒ 1 mg = 10-6 kg
1 mg = 1 mg ×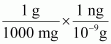
⇒ 1 mg = 106 ng
∴ 1 mg = 10-6 kg = 106 ng
(b) 1 mL = 1 mL ×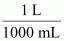
⇒ 1 mL = 10-3 L
1 mL = 1 cm3 = 1 cm3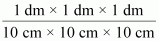
⇒ 1 mL = 10-3 dm3
∴ 1 mL = 10-3 L = 10-3 dm3
(i) 28.7 pm:
1 pm = 10–12 m
∴ 28.7 pm = 28.7 × 10–12 m
= 2.87 × 10–11 m
(ii) 15.15 pm:
1 pm = 10–12 m
∴ 15.15 pm = 15.15 × 10–12 m
= 1.515 × 10–11 m
(iii) 25365 mg:
1 mg = 10–3 g
25365 mg = 2.5365 × 104 × 10–3 g
Since,
1 g = 10–3 kg
2.5365 × 101 g = 2.5365 × 101 × 10–3 kg
∴ 25365 mg = 2.5365 × 10–2 kg
28:
Which one of the following will have largest number of atoms?
(i) 1 g Au (s)
(ii) 1 g Na (s)
(iii) 1 g Li (s)
(iv) 1 g of Cl2(g)
() Given,
Weight of 10.0L of the gas (at S.T.P) = 11.6 g
∴ Weight of 22.4 L of gas at STP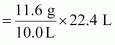
= 25.984 g
≈ 26 g
Hence, the molar mass of the gas is 26 g.
() Empirical formula mass of CH = 12 + 1 = 13 g
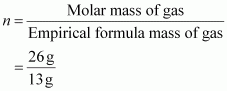
n = 2
∴ Molecular formula of gas = (CH)n
= C2H2
35:
Calcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 according to the reaction, CaCO3(s) + 2 HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
What mass of CaCO3 is required to react completely with 25 mL of 0.75 M HCl?
(a) If we fix the mass of dinitrogen at 28 g, then the masses of dioxygen that will combine with the fixed mass of dinitrogen are 32 g, 64 g, 32 g, and 80 g.
The masses of dioxygen bear a whole number ratio of 1:2:2:5. Hence, the given experimental data obeys the law of multiple proportions. The law states that if two elements combine to form more than one compound, then the masses of one element that combines with the fixed mass of another element are in the ratio of small whole numbers.
(b) null (b) 1 km = 1 km ×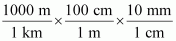
∴ 1 km = 106 mm
1 km = 1 km ×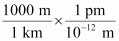
∴ 1 km = 1015 pm
Hence, 1 km = 106 mm = 1015 pm
(b) 1 mg = 1 mg ×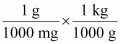
⇒ 1 mg = 10-6 kg
1 mg = 1 mg ×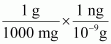
⇒ 1 mg = 106 ng
∴ 1 mg = 10-6 kg = 106 ng
(b) 1 mL = 1 mL ×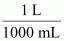
⇒ 1 mL = 10-3 L
1 mL = 1 cm3 = 1 cm3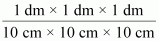
⇒ 1 mL = 10-3 dm3
∴ 1 mL = 10-3 L = 10-3 dm3
(i) 28.7 pm:
1 pm = 10–12 m
∴ 28.7 pm = 28.7 × 10–12 m
= 2.87 × 10–11 m
(ii) 15.15 pm:
1 pm = 10–12 m
∴ 15.15 pm = 15.15 × 10–12 m
= 1.515 × 10–11 m
(iii) 25365 mg:
1 mg = 10–3 g
25365 mg = 2.5365 × 104 × 10–3 g
Since,
1 g = 10–3 kg
2.5365 × 101 g = 2.5365 × 101 × 10–3 kg
∴ 25365 mg = 2.5365 × 10–2 kg
28:
Which one of the following will have largest number of atoms?
(i) 1 g Au (s)
(ii) 1 g Na (s)
(iii) 1 g Li (s)
(iv) 1 g of Cl2(g)
() Given,
Weight of 10.0L of the gas (at S.T.P) = 11.6 g
∴ Weight of 22.4 L of gas at STP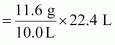
= 25.984 g
≈ 26 g
Hence, the molar mass of the gas is 26 g.
() Empirical formula mass of CH = 12 + 1 = 13 g
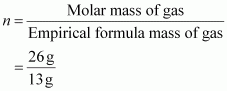
n = 2
∴ Molecular formula of gas = (CH)n
= C2H2
35:
Calcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 according to the reaction, CaCO3(s) + 2 HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
What mass of CaCO3 is required to react completely with 25 mL of 0.75 M HCl?
(a) If we fix the mass of dinitrogen at 28 g, then the masses of dioxygen that will combine with the fixed mass of dinitrogen are 32 g, 64 g, 32 g, and 80 g.
The masses of dioxygen bear a whole number ratio of 1:2:2:5. Hence, the given experimental data obeys the law of multiple proportions. The law states that if two elements combine to form more than one compound, then the masses of one element that combines with the fixed mass of another element are in the ratio of small whole numbers.
(b) null (b) 1 km = 1 km ×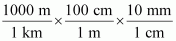
∴ 1 km = 106 mm
1 km = 1 km ×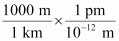
∴ 1 km = 1015 pm
Hence, 1 km = 106 mm = 1015 pm
(b) 1 mg = 1 mg ×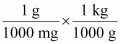
⇒ 1 mg = 10-6 kg
1 mg = 1 mg ×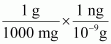
⇒ 1 mg = 106 ng
∴ 1 mg = 10-6 kg = 106 ng
(b) 1 mL = 1 mL ×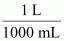
⇒ 1 mL = 10-3 L
1 mL = 1 cm3 = 1 cm3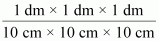
⇒ 1 mL = 10-3 dm3
∴ 1 mL = 10-3 L = 10-3 dm3
(i) 28.7 pm:
1 pm = 10–12 m
∴ 28.7 pm = 28.7 × 10–12 m
= 2.87 × 10–11 m
(ii) 15.15 pm:
1 pm = 10–12 m
∴ 15.15 pm = 15.15 × 10–12 m
= 1.515 × 10–11 m
(iii) 25365 mg:
1 mg = 10–3 g
25365 mg = 2.5365 × 104 × 10–3 g
Since,
1 g = 10–3 kg
2.5365 × 101 g = 2.5365 × 101 × 10–3 kg
∴ 25365 mg = 2.5365 × 10–2 kg
28:
Which one of the following will have largest number of atoms?
(i) 1 g Au (s)
(ii) 1 g Na (s)
(iii) 1 g Li (s)
(iv) 1 g of Cl2(g)
- #21-ii14 g 32 gAns : Given,
Weight of 10.0L of the gas (at S.T.P) = 11.6 g
∴ Weight of 22.4 L of gas at STP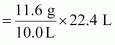
= 25.984 g
≈ 26 g
Hence, the molar mass of the gas is 26 g.
- #21-iii28 g 32 g
(iv) 28 g 80 gAns : Empirical formula mass of CH = 12 + 1 = 13 g
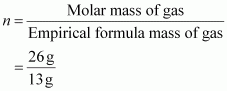
n = 2
∴ Molecular formula of gas = (CH)n
= C2H2
35:
Calcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 according to the reaction, CaCO3(s) + 2 HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
What mass of CaCO3 is required to react completely with 25 mL of 0.75 M HCl?
- #21-aWhich law of chemical combination is obeyed by the above experimental data?Give its statement.Ans : If we fix the mass of dinitrogen at 28 g, then the masses of dioxygen that will combine with the fixed mass of dinitrogen are 32 g, 64 g, 32 g, and 80 g.
The masses of dioxygen bear a whole number ratio of 1:2:2:5. Hence, the given experimental data obeys the law of multiple proportions. The law states that if two elements combine to form more than one compound, then the masses of one element that combines with the fixed mass of another element are in the ratio of small whole numbers.
- #21-bFill in the blanks in the following conversions:
- #21-b-i1 km = ...................... mm = ...................... pmAns : 1 km = 1 km ×
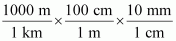
∴ 1 km = 106 mm
1 km = 1 km ×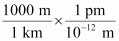
∴ 1 km = 1015 pm
Hence, 1 km = 106 mm = 1015 pm
- #21-b-ii1 mg = ...................... kg = ...................... ngAns : 1 mg = 1 mg ×
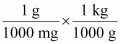
⇒ 1 mg = 10-6 kg
1 mg = 1 mg ×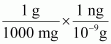
⇒ 1 mg = 106 ng
∴ 1 mg = 10-6 kg = 106 ng
- #21-b-iii1 mL = ...................... L = ...................... dm3Ans : 1 mL = 1 mL ×
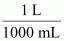
⇒ 1 mL = 10-3 L
1 mL = 1 cm3 = 1 cm3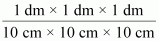
⇒ 1 mL = 10-3 dm3
∴ 1 mL = 10-3 L = 10-3 dm3
(i) 28.7 pm:
1 pm = 10–12 m
∴ 28.7 pm = 28.7 × 10–12 m
= 2.87 × 10–11 m
(ii) 15.15 pm:
1 pm = 10–12 m
∴ 15.15 pm = 15.15 × 10–12 m
= 1.515 × 10–11 m
(iii) 25365 mg:
1 mg = 10–3 g
25365 mg = 2.5365 × 104 × 10–3 g
Since,
1 g = 10–3 kg
2.5365 × 101 g = 2.5365 × 101 × 10–3 kg
∴ 25365 mg = 2.5365 × 10–2 kg
28:
Which one of the following will have largest number of atoms?
(i) 1 g Au (s)
(ii) 1 g Na (s)
(iii) 1 g Li (s)
(iv) 1 g of Cl2(g)