NEET-XI-Chemistry
01: Some Basic Concepts of Chemistry
- #17-iExpress this in percent by mass.
() Determine the molality of chloroform in the water sample.Ans : 1 ppm is equivalent to 1 part out of 1 million (106) parts.
∴ Mass percent of 15 ppm chloroform in water
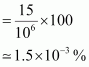
() 100 g of the sample contains 1.5 × 10-3 g of CHCl3.
⇒ 1000 g of the sample contains 1.5 × 10-2 g of CHCl3.
∴ Molality of chloroform in water
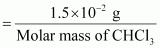
Molar mass of CHCl3 = 12.00 + 1.00 + 3(35.5)
= 119.5 g mol-1
∴ Molality of chloroform in water = 0.0125 × 10-2 m
= 1.25 × 10-4 m
- #17-iiDetermine the molality of chloroform in the water sample.Ans : 100 g of the sample contains 1.5 × 10-3 g of CHCl3.
⇒ 1000 g of the sample contains 1.5 × 10-2 g of CHCl3.
∴ Molality of chloroform in water
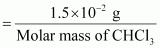
Molar mass of CHCl3 = 12.00 + 1.00 + 3(35.5)
= 119.5 g mol-1
∴ Molality of chloroform in water = 0.0125 × 10-2 m
= 1.25 × 10-4 m