ICSE-X-Chemistry
Previous Year Paper year:2009
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- #4-b-iiii. Which solution will give a gelatinous white precipitate with zinc sulphate solution?
The precipitate disappears when an excess of the solution is added.Ans : Solution C
- #4-b-iiiiii. Which solution could be a solution of glacial acetic acid?Ans : Solution B
- #4-b-iviv. Give an example of a solution which is a weak alkali.Ans : Ammonium hyroxide solution.
- #5
- #5-a [4]The diagram given below is to prepare iron(III) chloride in the laboratory:
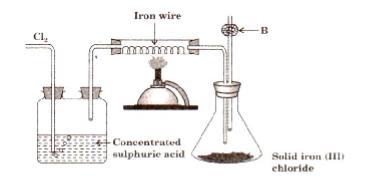
- #5-a-ii. What is substance B?Ans : B is anhydrous calcium chloride.
- #5-a-iiii. What is the purpose of B?Ans : B acts as a drying agent and removes moisture from the flask.
- #5-a-iiiiii. Why is iron (III) chloride to be stored in a closed container?Ans : Because iron (III) chloride is a deliquescent substance.
- #5-a-iviv. Write the equation for the reaction between iron and chlorine.Ans : $$\ce{2Fe + 3Cl2 -> 2FeCl3 } $$
- #5-bAns :

- #5-b-i [4]i. Write the equation(s) for the reaction(s) to prepare lead sulphate from lead
carbonate.
- #5-b-iiii. Methane, the first member of alkanes, when treated with excess of chlorine in the
presence of diffused sunlight forms carbon tetrachloride. Draw the appropriate
structural formula of carbon tetrachloride and state the type of bond present in it.
- #5-c [2]Aqueous solution of nickel sulphate contains ``\ce{Ni^{2+}}`` and ``\ce{SO4^{2-}}`` ions.
- #5-c-ii. Which ion moves towards the cathode?Ans : Ni2+ ions
- #5-c-iiii. What is the product at the anode?Ans : Oxygen gas when inert electrode is used.