ICSE-X-Chemistry
Previous Year Paper year:2009
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- #6
- #6-a [3]Give one chemical test to distinguish between the following pairs of compounds.
- #6-a-ii. Zinc sulphate solution and zinc chloride solution.Ans : On addition of BaCl2 solution, Zinc sulphate solution will give a white ppt. of BaSO4 while Zinc chloride solution will not give any ppt with BaCl2 solution.
- #6-a-iiii. Iron (II) chloride solution and iron (III) chloride solution.Ans : Iron II chloride gives a dirty green ppt with NaOH solution. Iron III chloride will give a reddish brown ppt with NaOH solution.
- #6-a-iiiiii. Calcium nitrate solution and calcium chloride solution.Ans : Calcium chloride solution will give a white ppt with AgNO3 solution while calcium nitrate solution will not give any ppt with AgNO3 solution.
- #6-b [3]Define the following terms:
- #6-b-ii. MoleAns : Mole: It is the quantity of a substance which contains Avogadro's number of constituent particles.
- #6-b-iiii. NeutralisationAns : Neutralisation: It is the process by which H+ ions of an acid react completely with the OH- ions of a base to give salt and water only.
- #6-b-iiiiii. Ionisation potentialAns : Ionisation Potential: It is the amount of energy required to remove a valence electron from an isolated gaseous atom of an element.
- #6-c [4]Fill in the blanks with the correct words from the brackets:
Generally, ionic compounds exist in (i) _______ (solid/liquid/gas) state. Melting and
boiling points of covalent compounds are generally (ii) _________ (low/high). The general
formula for alkane is (iii) __________ (``\ce{ C_nH_{2n}/C_nH_{2n-2}/C_nH_{2n+2}}``). For alkynes, the general
formula is (iv) _________ (``\ce{ C_nH_{2n}/C_nH_{2n-2}/C_nH_{2n+2}}``).Ans :
(i) solid
(ii) low
(iii) CnH2n+2
(iv) CnH2n-2
- #7
- #7-a [4]Give chemical equation forAns :
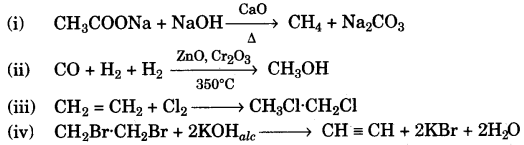
- #7-a-ii. The laboratory preparation of methane from sodium acetate.
- #7-a-iiii. The industrial preparation of methanol from water gas. **
- #7-a-iiiiii. The reaction of one mole of ethene with one mole of chlorine gas.