ICSE-X-Chemistry
Previous Year Paper year:2012
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- #1-c [5]Some word/words are missing in the following statements. You are required to rewrite
the statements in the correct form using the appropriate word/words:
- #1-c-ii. Ethyl alcohol is dehydrated by sulphuric acid at a temperature of about 170°C.Ans : Ethyl alcohol is dehydrated by concentrated sulphuric acid at a temperature of about 170°C.
- #1-c-iiii. Aqua regia contains one part by volume of nitric acid and three parts by volume of
hydrochloric acid.Ans : Aqua regia contains one part by volume of concentrated nitric acid and three parts by volume of concentrated hydrochloric acid.
- #1-c-iiiiii. Magnesium nitride reacts with water to liberate ammonia.Ans : Magnesium nitride reacts with boiling water to liberate ammonia.
- #1-c-iviv. Cations migrate during electrolysis.Ans : Cations migrate to cathode dining electrolysis.
- #1-c-vv. Magnesium reacts with nitric acid to liberate hydrogen gas.Ans : Magnesium reacts with very dilute nitric acid to liberate hydrogen gas.
- #1-d [5]Choose the correct answer from the options given below:
- #1-d-ii. An element in period 3 whose electron affinity is zero.
A) Neon B) Sulphur
C) Sodium D) ArgonAns : D-Argon
- #1-d-iiii. An alkaline earth metal.
A) Potassium B) Calcium
C) Lead D) CopperAns : B-Calcium
- #1-d-iiiiii. The vapour density of carbon dioxide [C = 12, 0 = 16]
A) 32 (B) 16
C) 44 (D) 22Ans : D-22
- #1-d-iviv. Identify the weak electrolyte from the following:
A) Sodium chloride solution (B) Dilute hydrochloric acid
C) Dilute sulphuric acid (D) Aqueous acetic acidAns : D-Aqueous acetic acid
- #1-d-vv. Which of the following metallic oxides cannot be reduced by normal reducing
agents?
A) Magnesium oxide (B) Copper (II) oxide
C) Zinc oxide (D) Iron (III) oxideAns : A-Magnesium oxide
- #1-e [5]Match the following:
Column A Column B (1) Acid salt (A) Ferrous ammonium sulphate (2) Double salt (B) Contains only ions (3)Ammonium hydroxide solution (C)Sodium hydrogen sulphate (4)Dilute hydrochloric acid (D) Contains only molecules (5) Carbon tetrachloride (E) Contains ions and molecules Ans :Column A Column B 1. Acid salt C. Sodium hydrogen sulphate 2. Double salt A. Ferrous ammonium Sulphate 3. Ammonium hydroxide solution E. Contains ions and molecules 4. Dilute hydrochloric acid B. Contains only ions 5. Carbon tetrachloride D. Contains only molecules
- #1-f [5]Give the structural formula for the following:Ans :
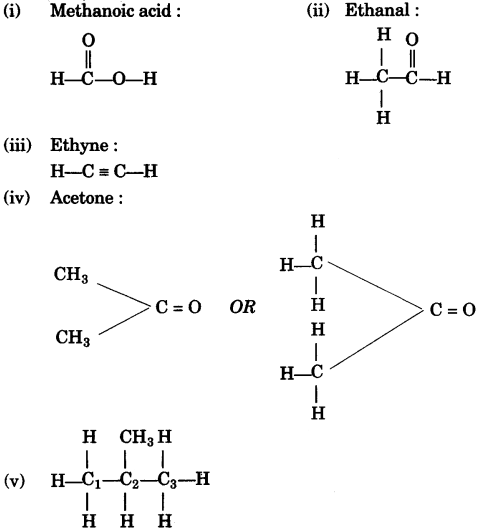
- #1-f-ii. Methanoic acid