ICSE-X-Chemistry
Previous Year Paper year:2012
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- #1-f-iiii. Ethanal
- #1-f-iiiiii. Ethyne
- #1-f-iviv. Acetone
- #1-f-vv. 2-methyl propane
- #1-gConcentrated nitric acid oxidises phosphorus to phosphoric acid according to the
following equation:
$$\ce{P + 5HNO3 (conc.) ->H3PO4 + H2O + 5NO2 }$$
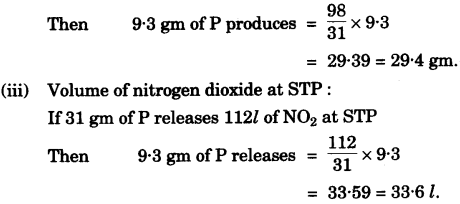
If ``\pu{9.3 g }`` of phosphorus was used in the reaction, then calculate:Ans :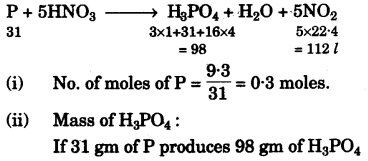
- #1-g-i [1]i. Number of moles of phosphorus taken.
- #1-g-ii [2]ii. The mass of phosphoric acid formed.
- #1-g-iii [2]iii. The volume of nitrogen dioxide produced at STP.
[H = 1, N = 14, P = 31, O = 16]
- #1-h [5]Give reasons for the following:
- #1-h-ii. Iron is rendered passive with fuming nitric acid.Ans : Cone. ``\ce{HNO_3}`` being a strong oxidising agent oxidises iron, forming a layer that makes iron non reactive or passive.
- #1-h-iiii. An aqueous solution of sodium chloride conducts electricity.Ans : Aqueous solution of sodium chloride contains mobile ions like ``\ce{Na+, Cl-, H+, OH-, H3O+}`` etc. so they conduct electricity.
- #1-h-iiiiii. Ionisation potential of the element increases across a period.Ans : Atomic size decreases and nuclear charges increases as we move from left to right in a period so energy required to remove one electron from the valence shell increases from left to right thus ionisation potential increases.
- #1-h-iviv. Alkali metals are good reducing agents.Ans : Alkali metals readily lose electrons from their valence shell and get oxidised. So they behave as good reducing agents.
- #1-h-vv. Hydrogen chloride gas cannot be dried over quick lime.Ans : Hydrogen chloride is acidic whereas quick lime is basic. So they will react with each other hence quick lime can not be used to dry hydrogen chloride.
- # [40]Section : IIAttempt any four questions from this section.