NEET-XII-Chemistry
06: Polymers
- Qstn #14How does the presence of double bonds in rubber molecules influence their
structure and reactivity?
Ans : Natural rubber is a linear cis-polyisoprene in which the double bonds are present between C2 and C3 of the isoprene units.
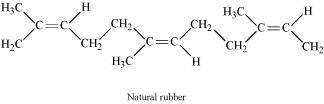
Because of this cis-configuration, intermolecular interactions between the various strands of isoprene are quite weak. As a result, various strands in natural rubber are arranged randomly. Hence, it shows elasticity.
- Qstn #15Discuss the main purpose of vulcanisation of rubber.
Ans : Natural rubber though useful has some problems associated with its use. These limitations are discussed below:
1. Natural rubber is quite soft and sticky at room temperature. At elevated temperatures (> 335 K), it becomes even softer. At low temperatures (< 283 K), it becomes brittle. Thus, to maintain its elasticity, natural rubber is generally used in the temperature range of 283 K-335 K.
2. It has the capacity to absorb large amounts of water.
3. It has low tensile strength and low resistance to abrasion.
4. It is soluble in non-polar solvents.
5. It is easily attacked by oxidizing agents.
Vulcanization of natural rubber is done to improve upon all these properties. In this process, a mixture of raw rubber with sulphur and appropriate additive is heated at a temperature range between 373 K and 415 K.
- Qstn #16What are the monomeric repeating units of Nylon-6 and Nylon-6, 6?
Ans : The monomeric repeating unit of nylon 6 is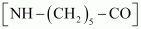 , which is derived from Caprolactam.
, which is derived from Caprolactam.
The monomeric repeating unit of nylon 6, 6 is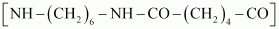 , which is derived from hexamethylene diamine and adipic acid.
, which is derived from hexamethylene diamine and adipic acid.
- Qstn #17Write the names and structures of the monomers of the following polymers:
() Buna-S () Buna-N
() Dacron () Neoprene
() Buna-N
() Dacron () Neoprene
() Buna-S () Buna-N
() Dacron () Neoprene
() Buna-N
() Dacron () NeopreneAns :-
Polymer
Monomer
Structure of monomer
i
Buna-S
1, 3-butadiene
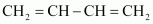
Styrene
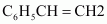
ii
Buna-N
1, 3-butadiene
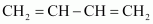
Acrylonitrile
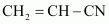
iii
Neoprene
Chloroprene
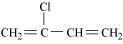
iv
Dacron
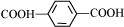
Ethylene glycol
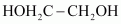
Terephthalic acid
-
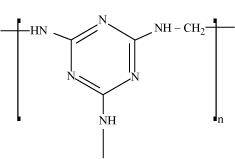
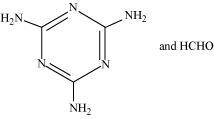
- Qstn #18Identify the monomer in the following polymeric structures.
()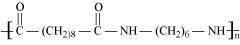
()
()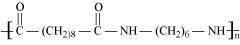
()
Ans : null () The monomers of the given polymeric structure are decanoic acid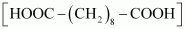 and hexamethylene diamine
and hexamethylene diamine 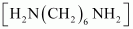 .
.
() The monomers of the given polymeric structure are
() The monomers of the given polymeric structure are decanoic acid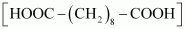 and hexamethylene diamine
and hexamethylene diamine 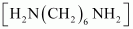 .
.
() The monomers of the given polymeric structure are

- Ans : The monomers of the given polymeric structure are decanoic acid
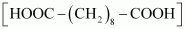 and hexamethylene diamine
and hexamethylene diamine 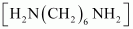 .
.
- Qstn #19How is dacron obtained from ethylene glycol and terephthalic acid?
Ans : The condensation polymerisation of ethylene glycol and terephthalic acid leads to the formation of dacron.
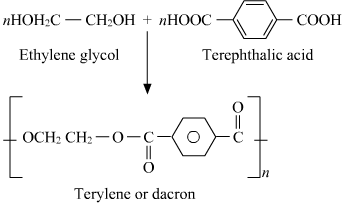
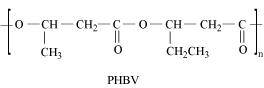
- Qstn #20What is a biodegradable polymer? Give an example of a biodegradable aliphatic polyester.
Ans : A polymer that can be decomposed by bacteria is called a biodegradable polymer.
Poly-β-hydroxybutyrate-CO-β- hydroxyvalerate (PHBV) is a biodegradable aliphatic polyester.