NEET-XII-Chemistry
09: Coordination Compounds
- #13Aqueous copper sulphate solution (blue in colour) gives:
() a green precipitate with aqueous potassium fluoride, and
() a bright green solution with aqueous potassium chloride
Explain these experimental results.
Ans : Aqueous CuSO4 exists as [Cu(H2O)4]SO4. It is blue in colour due to the presence of
[Cu[H2O)4]2+ ions.
() When KF is added:
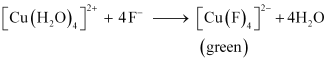
() When KCl is added:

In both these cases, the weak field ligand water is replaced by the F- and Cl- ions.
- #13-ia green precipitate with aqueous potassium fluoride, andAns : When KF is added:
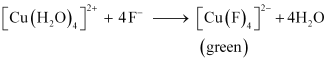
- #13-iia bright green solution with aqueous potassium chloride
Explain these experimental results.
Ans : When KCl is added:

In both these cases, the weak field ligand water is replaced by the F- and Cl- ions.