NEET-XII-Chemistry
09: Coordination Compounds
- #12Write all the geometrical isomers of [Pt(NH3)(Br)(Cl)(py)] and how many of these will exhibit optical isomers?
Ans : [Pt(NH3)(Br)(Cl)(py)
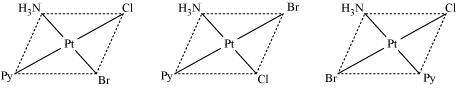
From the above isomers, none will exhibit optical isomers. Tetrahedral complexes rarely show optical isomerization. They do so only in the presence of unsymmetrical chelating agents.