NEET-XII-Chemistry
09: Coordination Compounds
- #11Draw all the isomers (geometrical and optical) of:
() [CoCl2(en)2]+
() [Co(NH3)Cl(en)2]2+
() [Co(NH3)2Cl2(en)]+
() [CoCl2(en)2]+
() [Co(NH3)Cl(en)2]2+
() [Co(NH3)2Cl2(en)]+Ans : null () [CoCl2(en)2]+ Geometrical isomerism
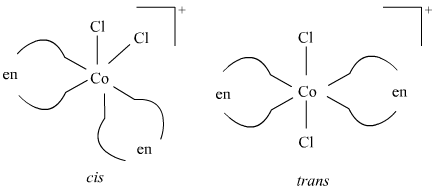
Optical isomerism Since only cis isomer is optically active, it shows optical isomerism.
.png)
In total, three isomers are possible.
() [Co(NH3)Cl(en)2]2+
Geometrical isomerism
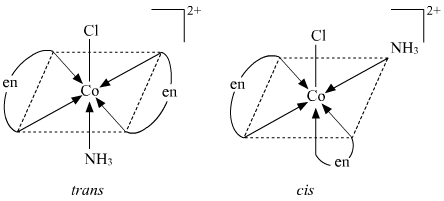
Optical isomerism Since only cis isomer is optically active, it shows optical isomerism.
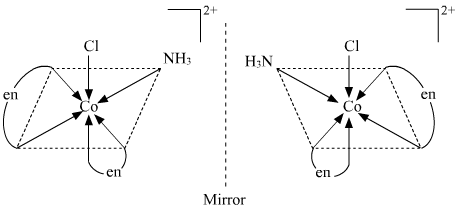
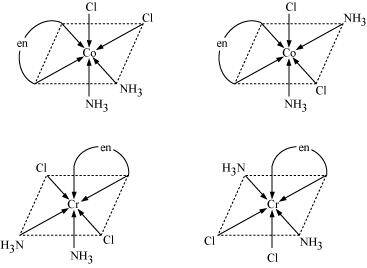
() [Co(NH3)2Cl2(en)]+
() [CoCl2(en)2]+ Geometrical isomerism

Optical isomerism Since only cis isomer is optically active, it shows optical isomerism.
.png)
In total, three isomers are possible.
() [Co(NH3)Cl(en)2]2+
Geometrical isomerism

Optical isomerism Since only cis isomer is optically active, it shows optical isomerism.

() [Co(NH3)2Cl2(en)]+

- #11-i[CoCl2(en)2]+Ans : [CoCl2(en)2]+ Geometrical isomerism

Optical isomerism Since only cis isomer is optically active, it shows optical isomerism.
.png)
In total, three isomers are possible.
- #11-ii[Co(NH3)Cl(en)2]2+Ans : [Co(NH3)Cl(en)2]2+
Geometrical isomerism

Optical isomerism Since only cis isomer is optically active, it shows optical isomerism.

- #11-iii[Co(NH3)2Cl2(en)]+Ans : [Co(NH3)2Cl2(en)]+
