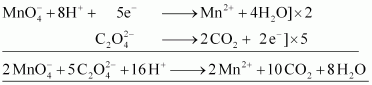NEET-XII-Chemistry
08: The d-and f-Block Elements
- #16-iiron(II) ions () SO2 and () oxalic acid?
Write the ionic equations for the reactions.
() SO2 and () oxalic acid?
Write the ionic equations for the reactions.
() SO2 and () oxalic acid?
Write the ionic equations for the reactions.
() SO2 and () oxalic acid?
Write the ionic equations for the reactions.
Ans : Acidified KMnO4 solution oxidizes Fe (II) ions to Fe (III) ions i.e., ferrous ions to ferric ions.

() Acidified potassium permanganate oxidizes SO2 to sulphuric acid.
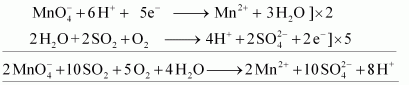
() Acidified potassium permanganate oxidizes oxalic acid to carbon dioxide.
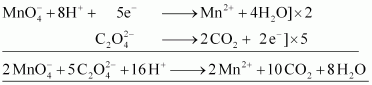
() Acidified potassium permanganate oxidizes SO2 to sulphuric acid.
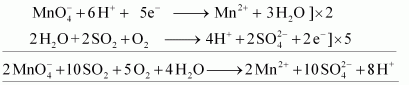
() Acidified potassium permanganate oxidizes oxalic acid to carbon dioxide.
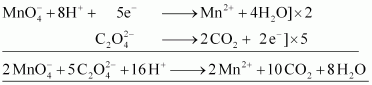
() Acidified potassium permanganate oxidizes SO2 to sulphuric acid.
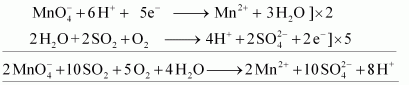
() Acidified potassium permanganate oxidizes oxalic acid to carbon dioxide.
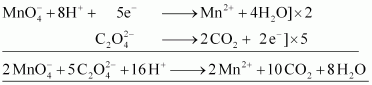
() Acidified potassium permanganate oxidizes SO2 to sulphuric acid.
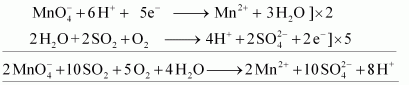
() Acidified potassium permanganate oxidizes oxalic acid to carbon dioxide.
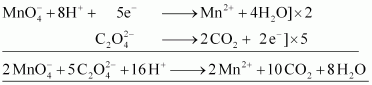
- #16-iiSO2 andAns : Acidified potassium permanganate oxidizes SO2 to sulphuric acid.
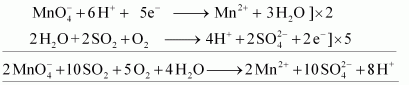
- #16-iiioxalic acid?
Write the ionic equations for the reactions.
Ans : Acidified potassium permanganate oxidizes oxalic acid to carbon dioxide.