NEET-XII-Chemistry
08: The d-and f-Block Elements
- #24Calculate the number of unpaired electrons in the following gaseous ions: Mn3+, Cr3+, V3+ and Ti3+. Which one of these is the most stable in aqueous solution?
Ans :-
Gaseous ions
Number of unpaired electrons
(i)
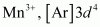
4
(ii)
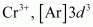
3
(iii)
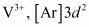
2
(vi)
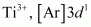
1
Cr3+ is the most stable in aqueous solutions owing to a.gif) configuration.
configuration.
-