NEET-XII-Chemistry
07: The p-Block Elements
- Qstn #34With what neutral molecule is ClO- isoelectronic? Is that molecule a Lewis base?
Ans : ClO- is isoelectronic to ClF. Also, both species contain 26 electrons in all as shown.
Total electrons ClO- = 17 + 8 + 1 = 26
In ClF = 17 + 9 = 26
ClF acts like a Lewis base as it accepts electrons from F to form ClF3.
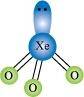
- Qstn #35How are XeO3 and XeOF4 prepared?
Ans : (i) XeO3 can be prepared in two ways as shown.
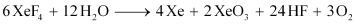

(ii) XeOF4 can be prepared using XeF6.

- Qstn #36-iF2, Cl2, Br2, I2 - increasing bond dissociation enthalpy.Ans : Bond dissociation energy usually decreases on moving down a group as the atomic size increases. However, the bond dissociation energy of F2 is lower than that of Cl2 and Br2. This is due to the small atomic size of fluorine. Thus, the increasing order for bond dissociation energy among halogens is as follows:
I2 < F2 < Br2 < Cl2
- Qstn #36-iiHF, HCl, HBr, HI - increasing acid strength.Ans : HF < HCl < HBr < HI
The bond dissociation energy of H-X molecules where X = F, Cl, Br, I, decreases with an increase in the atomic size. Since H-I bond is the weakest, HI is the strongest acid.
- Qstn #36-iiiNH3, PH3, AsH3, SbH3, BiH3 - increasing base strength.Ans : BiH3 ≤ SbH3 < AsH3 < PH3 < NH3
On moving from nitrogen to bismuth, the size of the atom increases while the electron density on the atom decreases. Thus, the basic strength decreases.
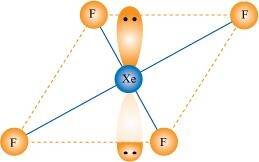
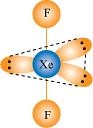
- Qstn #38Give the formula and describe the structure of a noble gas species which is isostructural with:
- Qstn #39Why do noble gases have comparatively large atomic sizes?
Ans : Noble gases do not form molecules. In case of noble gases, the atomic radii corresponds to van der Waal’s radii. On the other hand, the atomic radii of other elements correspond to their covalent radii. By definition, van der Waal’s radii are larger than covalent radii. It is for this reason that noble gases are very large in size as compared to other atoms belonging to the same period.
- Qstn #40List the uses of Neon and argon gases.
Ans : Uses of neon gas:
(i) It is mixed with helium to protect electrical equipments from high voltage.
(ii) It is filled in discharge tubes with characteristic colours.
(iii) It is used in beacon lights.
Uses of Argon gas:
(i) Argon along with nitrogen is used in gas-filled electric lamps. This is because Ar is more inert than N.
(ii) It is usually used to provide an inert temperature in a high metallurgical process.
(iii) It is also used in laboratories to handle air-sensitive substances.