NEET-XII-Chemistry
07: The p-Block Elements
- Qstn #22How is SO2 an air pollutant?
Ans : Sulphur dioxide causes harm to the environment in many ways:
1. It combines with water vapour present in the atmosphere to form sulphuric acid. This causes acid rain. Acid rain damages soil, plants, and buildings, especially those made of marble.
2. Even in very low concentrations, SO2 causes irritation in the respiratory tract. It causes throat and eye irritation and can also affect the larynx to cause breathlessness.
3. It is extremely harmful to plants. Plants exposed to sulphur dioxide for a long time lose colour from their leaves. This condition is known as chlorosis. This happens because the formation of chlorophyll is affected by the presence of sulphur dioxide.
- Qstn #23Why are halogens strong oxidising agents?
Ans : The general electronic configuration of halogens is np5, where n = 2-6. Thus, halogens need only one more electron to complete their octet and to attain the stable noble gas configuration. Also, halogens are highly electronegative with low dissociation energies and high negative electron gain enthalpies. Therefore, they have a high tendency to gain an electron. Hence, they act as strong oxidizing agents.
- Qstn #24Explain why fluorine forms only one oxoacid, HOF.
Ans : Fluorine forms only one oxoacid i.e., HOF because of its high electronegativity and small size.
- Qstn #25Explain why inspite of nearly the same electronegativity, oxygen forms hydrogen bonding while chlorine does not.
Ans : Both chlorine and oxygen have almost the same electronegativity values, but chlorine rarely forms hydrogen bonding. This is because in comparison to chlorine, oxygen has a smaller size and as a result, a higher electron density per unit volume.
- Qstn #26Write two uses of ClO2.
Ans : Uses of ClO2:
(i) It is used for purifying water.
(ii) It is used as a bleaching agent.
- Qstn #27Why are halogens coloured?
Ans : Almost all halogens are coloured. This is because halogens absorb radiations in the visible region. This results in the excitation of valence electrons to a higher energy region. Since the amount of energy required for excitation differs for each halogen, each halogen displays a different colour.
- Qstn #29How can you prepare Cl2 from HCl and HCl from Cl2? Write reactions only.
Ans : (i) Cl2 can be prepared from HCl by Deacon’s process.

(ii) HCl can be prepared from Cl2 on treating it with water.
.gif)
- Qstn #30What inspired N. Bartlett for carrying out reaction between Xe and PtF6?
Ans : Neil Bartlett initially carried out a reaction between oxygen and PtF6. This resulted in the formation of a red compound, .
.
Later, he realized that the first ionization energy of oxygen (1175 kJ/mol) and Xe (1170 kJ/mol) is almost the same. Thus, he tried to prepare a compound with Xe and PtF6. He was successful and a red-coloured compound, was formed.
was formed.
- Qstn #31-iH3PO3 (ii) PCl3 (iii) Ca3P2
(iv) Na3PO4 (v) POF3?
Ans : H3PO3
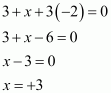
(ii) PCl3
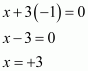
(iii) Ca3P2
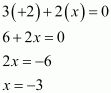
(iv) Na3PO4
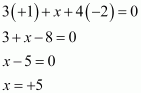
(v) POF3
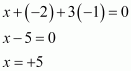
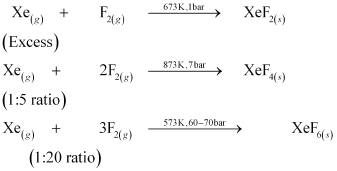
- Qstn #33How are xenon fluorides XeF2, XeF4 and XeF6 obtained?
Ans : XeF2, XeF4, and XeF6 are obtained by a direct reaction between Xe and F2. The condition under which the reaction is carried out determines the product.