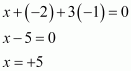NEET-XII-Chemistry
07: The p-Block Elements
- #31What are the oxidation states of phosphorus in the following:
() H3PO3 (ii) PCl3 (iii) Ca3P2
(iv) Na3PO4 (v) POF3?
() H3PO3 (ii) PCl3 (iii) Ca3P2
(iv) Na3PO4 (v) POF3?
Ans :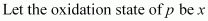 .
.
() H3PO3
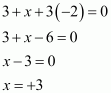
(ii) PCl3
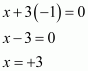
(iii) Ca3P2
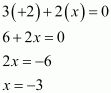
(iv) Na3PO4
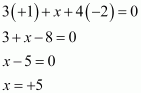
(v) POF3
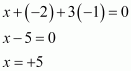
() H3PO3
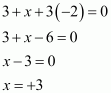
(ii) PCl3
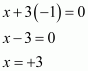
(iii) Ca3P2
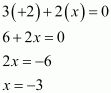
(iv) Na3PO4
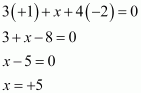
(v) POF3
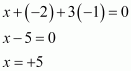
- #31-iH3PO3 (ii) PCl3 (iii) Ca3P2
(iv) Na3PO4 (v) POF3?
Ans : H3PO3
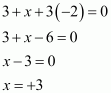
(ii) PCl3
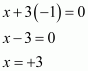
(iii) Ca3P2
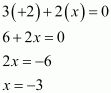
(iv) Na3PO4
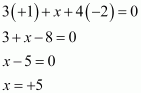
(v) POF3