NEET-XII-Chemistry
06: General Principles and Processes of Isolation of Elements
- #3Why is the extraction of copper from pyrites more difficult than that from its
oxide ore through reduction?
Ans : The Gibbs free energy of formation (ΔfG) of Cu2S is less than that of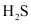 and
and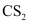 . Therefore, H2 and C cannot reduce Cu2S to Cu.
. Therefore, H2 and C cannot reduce Cu2S to Cu.
On the other hand, the Gibbs free energy of formation of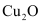 is greater than that of
is greater than that of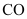 . Hence, C can reduce Cu2O to Cu.
. Hence, C can reduce Cu2O to Cu.
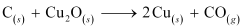
Hence, the extraction of copper from its pyrite ore is difficult than from its oxide ore through reduction.