NEET-XII-Chemistry
06: General Principles and Processes of Isolation of Elements
- #21The value of
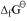 for formation of Cr2O3 is - 540 kJmol-1 and that of Al2 O3 is - 827 kJmol-1. Is the reduction of Cr2O3 possible with Al?
for formation of Cr2O3 is - 540 kJmol-1 and that of Al2 O3 is - 827 kJmol-1. Is the reduction of Cr2O3 possible with Al?
Ans : The value of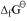 for the formation of Cr2O3 from Cr (-540 kJmol-1) is higher than that of Al2O3 from Al (-827 kJmol-1). Therefore, Al can reduce Cr2O3 to Cr. Hence, the reduction of Cr2O3 with Al is possible.
for the formation of Cr2O3 from Cr (-540 kJmol-1) is higher than that of Al2O3 from Al (-827 kJmol-1). Therefore, Al can reduce Cr2O3 to Cr. Hence, the reduction of Cr2O3 with Al is possible.
Alternatively,

Subtracting equation (ii) from (i), we have
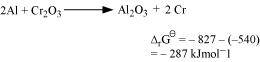
As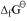 for the reduction reaction of Cr2O3 by Al is negative, this reaction is possible.
for the reduction reaction of Cr2O3 by Al is negative, this reaction is possible.