NEET-XII-Chemistry
06: General Principles and Processes of Isolation of Elements
- #23The choice of a reducing agent in a particular case depends on
thermodynamic factor. How far do you agree with this statement? Support
your opinion with two examples.
Ans :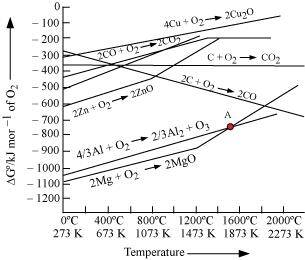
The above figure is a plot of Gibbs energy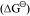 vs. T for formation of some oxides.
vs. T for formation of some oxides.
It can be observed from the above graph that a metal can reduce the oxide of other metals, if the standard free energy of formation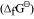 of the oxide of the former is more negative than the latter. For example, since
of the oxide of the former is more negative than the latter. For example, since 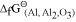 is more negative than
is more negative than 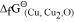 , Al can reduce Cu2O to Cu, but Cu cannot reduce Al2O3. Similarly, Mg can reduce ZnO to Zn, but Zn cannot reduce MgO because
, Al can reduce Cu2O to Cu, but Cu cannot reduce Al2O3. Similarly, Mg can reduce ZnO to Zn, but Zn cannot reduce MgO because 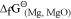 is more negative than
is more negative than 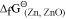 .
.