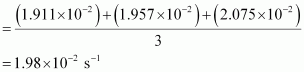NEET-XII-Chemistry
04: Chemical Kinetics
- #8In a pseudo first order hydrolysis of ester in water, the following results were obtained:
t/s
0
30
60
90
[Ester]mol L-1
0.55
0.31
0.17
0.085
() Calculate the average rate of reaction between the time interval 30 to 60 seconds.
() Calculate the pseudo first order rate constant for the hydrolysis of ester.
() Calculate the average rate of reaction between the time interval 30 to 60 seconds.
() Calculate the pseudo first order rate constant for the hydrolysis of ester.Ans : null () Average rate of reaction between the time interval, 30 to 60 seconds,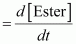
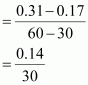
= 4.67 × 10-3 mol L-1 s-1
() For a pseudo first order reaction,
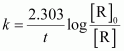
For t = 30 s,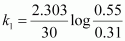
= 1.911 × 10-2 s-1
For t = 60 s,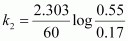
= 1.957 × 10-2 s-1
For t = 90 s,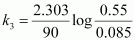
= 2.075 × 10-2 s-1
Then, average rate constant,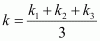
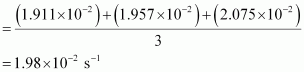
() Average rate of reaction between the time interval, 30 to 60 seconds,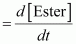
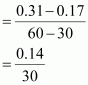
= 4.67 × 10-3 mol L-1 s-1
() For a pseudo first order reaction,
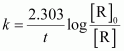
For t = 30 s,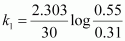
= 1.911 × 10-2 s-1
For t = 60 s,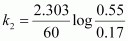
= 1.957 × 10-2 s-1
For t = 90 s,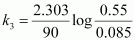
= 2.075 × 10-2 s-1
Then, average rate constant,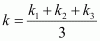
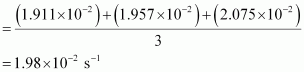
- #8-iCalculate the average rate of reaction between the time interval 30 to 60 seconds.Ans : Average rate of reaction between the time interval, 30 to 60 seconds,
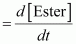
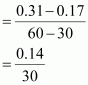
= 4.67 × 10-3 mol L-1 s-1
- #8-iiCalculate the pseudo first order rate constant for the hydrolysis of ester.Ans : For a pseudo first order reaction,
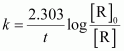
For t = 30 s,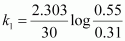
= 1.911 × 10-2 s-1
For t = 60 s,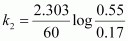
= 1.957 × 10-2 s-1
For t = 90 s,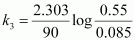
= 2.075 × 10-2 s-1
Then, average rate constant,