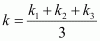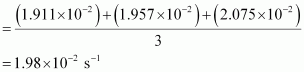NEET-XII-Chemistry
04: Chemical Kinetics
- #8In a pseudo first order hydrolysis of ester in water, the following results were obtained:
t/s
0
30
60
90
[Ester]mol L-1
0.55
0.31
0.17
0.085
() Calculate the average rate of reaction between the time interval 30 to 60 seconds.
() Calculate the pseudo first order rate constant for the hydrolysis of ester.Ans : null () Average rate of reaction between the time interval, 30 to 60 seconds,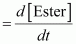
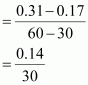
= 4.67 × 10-3 mol L-1 s-1
() For a pseudo first order reaction,
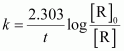
For t = 30 s,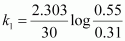
= 1.911 × 10-2 s-1
For t = 60 s,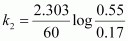
= 1.957 × 10-2 s-1
For t = 90 s,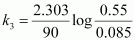
= 2.075 × 10-2 s-1
Then, average rate constant,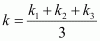
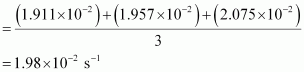
- #8-iCalculate the average rate of reaction between the time interval 30 to 60 seconds.Ans : Average rate of reaction between the time interval, 30 to 60 seconds,
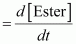
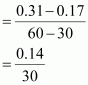
= 4.67 × 10-3 mol L-1 s-1
- #8-iiCalculate the pseudo first order rate constant for the hydrolysis of ester.Ans : For a pseudo first order reaction,
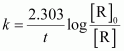
For t = 30 s,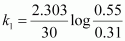
= 1.911 × 10-2 s-1
For t = 60 s,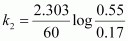
= 1.957 × 10-2 s-1
For t = 90 s,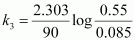
= 2.075 × 10-2 s-1
Then, average rate constant,