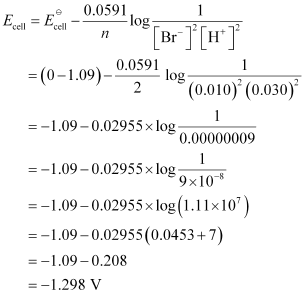NEET-XII-Chemistry
03: Electrochemistry
- #5Write the Nernst equation and emf of the following cells at 298 K:
() Mg(s) | Mg2+(0.001M) || Cu2+(0.0001 M) | Cu(s)
() Fe(s) | Fe2+(0.001M) || H+(1M)|H2(g)(1bar) | Pt(s)
() Sn(s) | Sn2+(0.050 M) || H+(0.020 M) | H2(g) (1 bar) | Pt(s)
() Pt(s) | Br2(l) | Br-(0.010 M) || H+(0.030 M) | H2(g) (1 bar) | Pt(s).
() Fe(s) | Fe2+(0.001M) || H+(1M)|H2(g)(1bar) | Pt(s)
() Sn(s) | Sn2+(0.050 M) || H+(0.020 M) | H2(g) (1 bar) | Pt(s)
() Pt(s) | Br2(l) | Br-(0.010 M) || H+(0.030 M) | H2(g) (1 bar) | Pt(s).
() Fe(s) | Fe2+(0.001M) || H+(1M)|H2(g)(1bar) | Pt(s)
() Sn(s) | Sn2+(0.050 M) || H+(0.020 M) | H2(g) (1 bar) | Pt(s)
() Pt(s) | Br2(l) | Br-(0.010 M) || H+(0.030 M) | H2(g) (1 bar) | Pt(s).
() Fe(s) | Fe2+(0.001M) || H+(1M)|H2(g)(1bar) | Pt(s)
() Sn(s) | Sn2+(0.050 M) || H+(0.020 M) | H2(g) (1 bar) | Pt(s)
() Pt(s) | Br2(l) | Br-(0.010 M) || H+(0.030 M) | H2(g) (1 bar) | Pt(s).
() Mg(s) | Mg2+(0.001M) || Cu2+(0.0001 M) | Cu(s)
() Fe(s) | Fe2+(0.001M) || H+(1M)|H2(g)(1bar) | Pt(s)
() Sn(s) | Sn2+(0.050 M) || H+(0.020 M) | H2(g) (1 bar) | Pt(s)
() Pt(s) | Br2(l) | Br-(0.010 M) || H+(0.030 M) | H2(g) (1 bar) | Pt(s).
() Fe(s) | Fe2+(0.001M) || H+(1M)|H2(g)(1bar) | Pt(s)
() Sn(s) | Sn2+(0.050 M) || H+(0.020 M) | H2(g) (1 bar) | Pt(s)
() Pt(s) | Br2(l) | Br-(0.010 M) || H+(0.030 M) | H2(g) (1 bar) | Pt(s).
() Fe(s) | Fe2+(0.001M) || H+(1M)|H2(g)(1bar) | Pt(s)
() Sn(s) | Sn2+(0.050 M) || H+(0.020 M) | H2(g) (1 bar) | Pt(s)
() Pt(s) | Br2(l) | Br-(0.010 M) || H+(0.030 M) | H2(g) (1 bar) | Pt(s).
() Fe(s) | Fe2+(0.001M) || H+(1M)|H2(g)(1bar) | Pt(s)
() Sn(s) | Sn2+(0.050 M) || H+(0.020 M) | H2(g) (1 bar) | Pt(s)
() Pt(s) | Br2(l) | Br-(0.010 M) || H+(0.030 M) | H2(g) (1 bar) | Pt(s).Ans : null () For the given reaction, the Nernst equation can be given as:
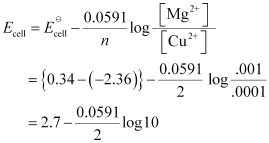
= 2.7 - 0.02955
= 2.67 V (approximately)
() For the given reaction, the Nernst equation can be given as:
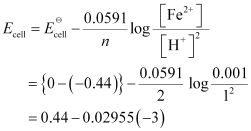
= 0.52865 V
= 0.53 V (approximately)
() For the given reaction, the Nernst equation can be given as:
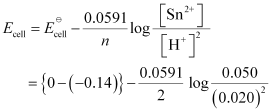
= 0.14 - 0.0295 × log125
= 0.14 - 0.062
= 0.078 V
= 0.08 V (approximately)
() For the given reaction, the Nernst equation can be given as:
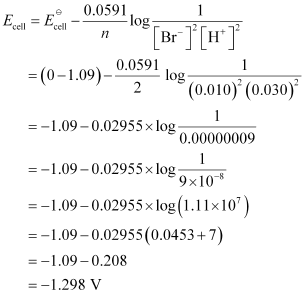
() For the given reaction, the Nernst equation can be given as:
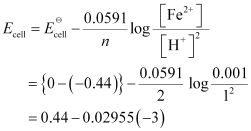
= 0.52865 V
= 0.53 V (approximately)
() For the given reaction, the Nernst equation can be given as:
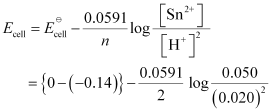
= 0.14 - 0.0295 × log125
= 0.14 - 0.062
= 0.078 V
= 0.08 V (approximately)
() For the given reaction, the Nernst equation can be given as:
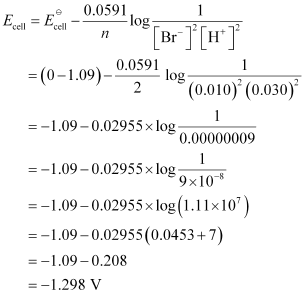
() For the given reaction, the Nernst equation can be given as:
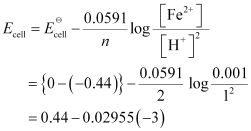
= 0.52865 V
= 0.53 V (approximately)
() For the given reaction, the Nernst equation can be given as:
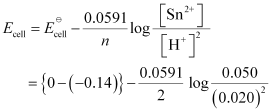
= 0.14 - 0.0295 × log125
= 0.14 - 0.062
= 0.078 V
= 0.08 V (approximately)
() For the given reaction, the Nernst equation can be given as:
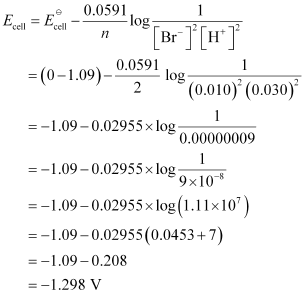
() For the given reaction, the Nernst equation can be given as:
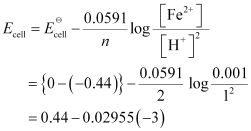
= 0.52865 V
= 0.53 V (approximately)
() For the given reaction, the Nernst equation can be given as:
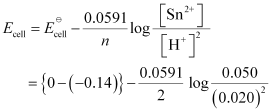
= 0.14 - 0.0295 × log125
= 0.14 - 0.062
= 0.078 V
= 0.08 V (approximately)
() For the given reaction, the Nernst equation can be given as:
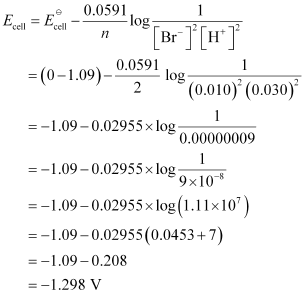
() For the given reaction, the Nernst equation can be given as:
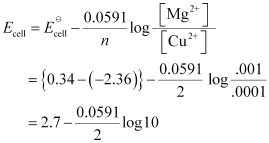
= 2.7 - 0.02955
= 2.67 V (approximately)
() For the given reaction, the Nernst equation can be given as:
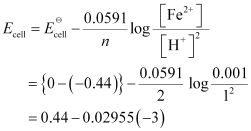
= 0.52865 V
= 0.53 V (approximately)
() For the given reaction, the Nernst equation can be given as:
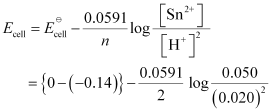
= 0.14 - 0.0295 × log125
= 0.14 - 0.062
= 0.078 V
= 0.08 V (approximately)
() For the given reaction, the Nernst equation can be given as:
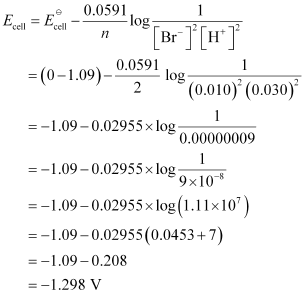
() For the given reaction, the Nernst equation can be given as:
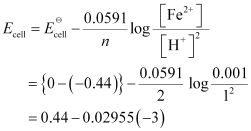
= 0.52865 V
= 0.53 V (approximately)
() For the given reaction, the Nernst equation can be given as:
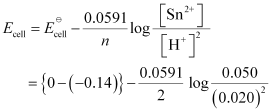
= 0.14 - 0.0295 × log125
= 0.14 - 0.062
= 0.078 V
= 0.08 V (approximately)
() For the given reaction, the Nernst equation can be given as:
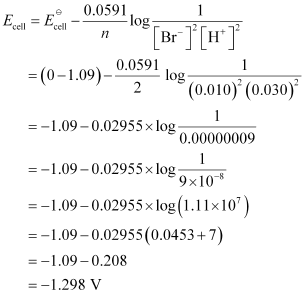
() For the given reaction, the Nernst equation can be given as:
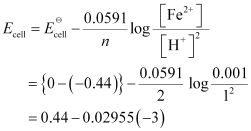
= 0.52865 V
= 0.53 V (approximately)
() For the given reaction, the Nernst equation can be given as:
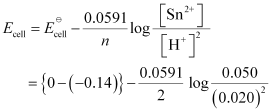
= 0.14 - 0.0295 × log125
= 0.14 - 0.062
= 0.078 V
= 0.08 V (approximately)
() For the given reaction, the Nernst equation can be given as:
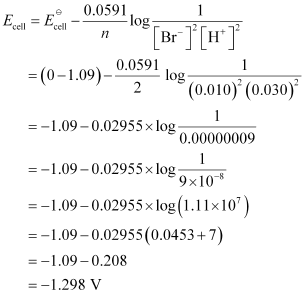
() For the given reaction, the Nernst equation can be given as:
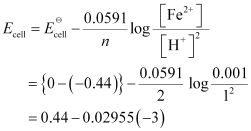
= 0.52865 V
= 0.53 V (approximately)
() For the given reaction, the Nernst equation can be given as:
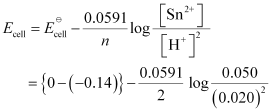
= 0.14 - 0.0295 × log125
= 0.14 - 0.062
= 0.078 V
= 0.08 V (approximately)
() For the given reaction, the Nernst equation can be given as:
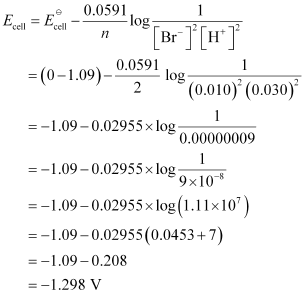
- #5-iMg(s) | Mg2+(0.001M) || Cu2+(0.0001 M) | Cu(s)
() Fe(s) | Fe2+(0.001M) || H+(1M)|H2(g)(1bar) | Pt(s)
() Sn(s) | Sn2+(0.050 M) || H+(0.020 M) | H2(g) (1 bar) | Pt(s)
() Pt(s) | Br2(l) | Br-(0.010 M) || H+(0.030 M) | H2(g) (1 bar) | Pt(s).
() Fe(s) | Fe2+(0.001M) || H+(1M)|H2(g)(1bar) | Pt(s)
() Sn(s) | Sn2+(0.050 M) || H+(0.020 M) | H2(g) (1 bar) | Pt(s)
() Pt(s) | Br2(l) | Br-(0.010 M) || H+(0.030 M) | H2(g) (1 bar) | Pt(s).
() Fe(s) | Fe2+(0.001M) || H+(1M)|H2(g)(1bar) | Pt(s)
() Sn(s) | Sn2+(0.050 M) || H+(0.020 M) | H2(g) (1 bar) | Pt(s)
() Pt(s) | Br2(l) | Br-(0.010 M) || H+(0.030 M) | H2(g) (1 bar) | Pt(s).Ans : For the given reaction, the Nernst equation can be given as:
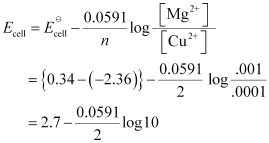
= 2.7 - 0.02955
= 2.67 V (approximately)
() For the given reaction, the Nernst equation can be given as:
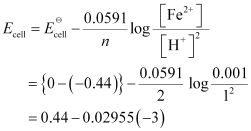
= 0.52865 V
= 0.53 V (approximately)
() For the given reaction, the Nernst equation can be given as:
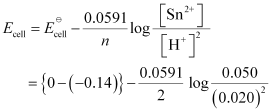
= 0.14 - 0.0295 × log125
= 0.14 - 0.062
= 0.078 V
= 0.08 V (approximately)
() For the given reaction, the Nernst equation can be given as:
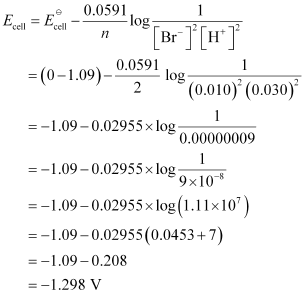
() For the given reaction, the Nernst equation can be given as:
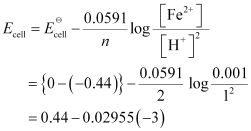
= 0.52865 V
= 0.53 V (approximately)
() For the given reaction, the Nernst equation can be given as:
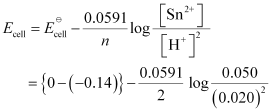
= 0.14 - 0.0295 × log125
= 0.14 - 0.062
= 0.078 V
= 0.08 V (approximately)
() For the given reaction, the Nernst equation can be given as:
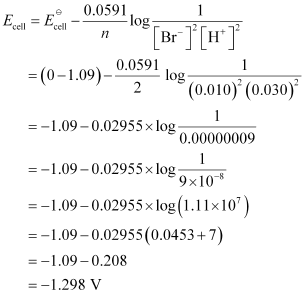
() For the given reaction, the Nernst equation can be given as:
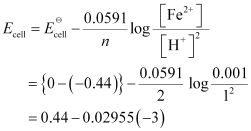
= 0.52865 V
= 0.53 V (approximately)
() For the given reaction, the Nernst equation can be given as:
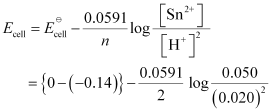
= 0.14 - 0.0295 × log125
= 0.14 - 0.062
= 0.078 V
= 0.08 V (approximately)
() For the given reaction, the Nernst equation can be given as:
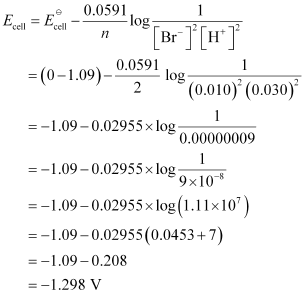
- #5-iiFe(s) | Fe2+(0.001M) || H+(1M)|H2(g)(1bar) | Pt(s)Ans : For the given reaction, the Nernst equation can be given as:
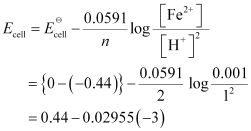
= 0.52865 V
= 0.53 V (approximately)
- #5-iiiSn(s) | Sn2+(0.050 M) || H+(0.020 M) | H2(g) (1 bar) | Pt(s)Ans : For the given reaction, the Nernst equation can be given as:
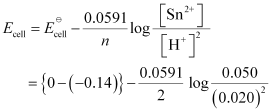
= 0.14 - 0.0295 × log125
= 0.14 - 0.062
= 0.078 V
= 0.08 V (approximately)
- #5-ivPt(s) | Br2(l) | Br-(0.010 M) || H+(0.030 M) | H2(g) (1 bar) | Pt(s).Ans : For the given reaction, the Nernst equation can be given as: