NEET-XII-Chemistry
03: Electrochemistry
- #4-i2Cr(s) + 3Cd2+(aq) → 2Cr3+(aq) + 3Cd
() Fe2+(aq) + Ag+(aq) → Fe3+(aq) + Ag(s)
Calculate the ΔrGθ and equilibrium constant of the reactions.
() Fe2+(aq) + Ag+(aq) → Fe3+(aq) + Ag(s)
Calculate the ΔrGθ and equilibrium constant of the reactions.
() Fe2+(aq) + Ag+(aq) → Fe3+(aq) + Ag(s)
Calculate the ΔrGθ and equilibrium constant of the reactions.
Ans :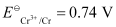
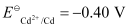
The galvanic cell of the given reaction is depicted as:
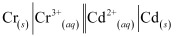
Now, the standard cell potential is
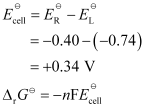
In the given equation,
n = 6
F = 96487 C mol-1
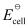 = +0.34 V
= +0.34 V
Then,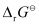 = -6 × 96487 C mol-1 × 0.34 V
= -6 × 96487 C mol-1 × 0.34 V
= -196833.48 CV mol-1
= -196833.48 J mol-1
= -196.83 kJ mol-1
Again,
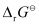 = -RT ln K
= -RT ln K
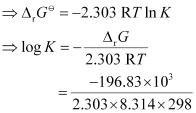
= 34.496
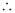 K = antilog (34.496)
K = antilog (34.496)
= 3.13 × 1034
()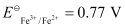
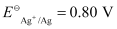
The galvanic cell of the given reaction is depicted as:
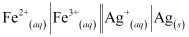
Now, the standard cell potential is
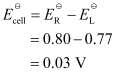
Here, n = 1.
Then,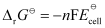
= -1 × 96487 C mol-1 × 0.03 V
= -2894.61 J mol-1
= -2.89 kJ mol-1
Again,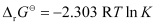
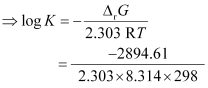
= 0.5073
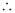 K = antilog (0.5073)
K = antilog (0.5073)
= 3.2 (approximately)
()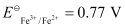
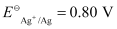
The galvanic cell of the given reaction is depicted as:
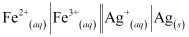
Now, the standard cell potential is
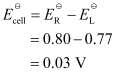
Here, n = 1.
Then,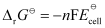
= -1 × 96487 C mol-1 × 0.03 V
= -2894.61 J mol-1
= -2.89 kJ mol-1
Again,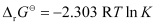
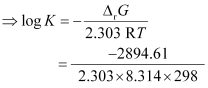
= 0.5073
 K = antilog (0.5073)
K = antilog (0.5073)
= 3.2 (approximately)
()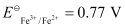
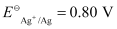
The galvanic cell of the given reaction is depicted as:
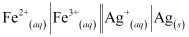
Now, the standard cell potential is
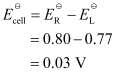
Here, n = 1.
Then,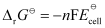
= -1 × 96487 C mol-1 × 0.03 V
= -2894.61 J mol-1
= -2.89 kJ mol-1
Again,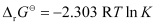
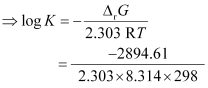
= 0.5073
 K = antilog (0.5073)
K = antilog (0.5073)
= 3.2 (approximately)
- #4-iiFe2+(aq) + Ag+(aq) → Fe3+(aq) + Ag(s)
Calculate the ΔrGθ and equilibrium constant of the reactions.
Ans :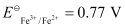
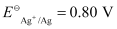
The galvanic cell of the given reaction is depicted as:
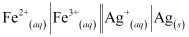
Now, the standard cell potential is
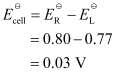
Here, n = 1.
Then,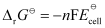
= -1 × 96487 C mol-1 × 0.03 V
= -2894.61 J mol-1
= -2.89 kJ mol-1
Again,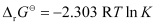
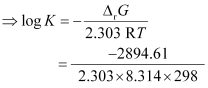
= 0.5073
 K = antilog (0.5073)
K = antilog (0.5073)
= 3.2 (approximately)