NEET-XII-Chemistry
02: Solutions
- #6H2S,
a toxic gas with rotten egg like smell, is used for the qualitative
analysis. If the solubility of H2S
in water at STP is 0.195 m, calculate Henry’s law constant.Ans : It
is given that the solubility of H2S
in water at STP is 0.195 m, i.e., 0.195 mol of H2S
is dissolved in 1000 g of water.
Moles
of water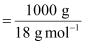
= 55.56
mol
∴Mole
fraction of H2S, x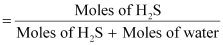
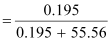
= 0.0035
At
STP, pressure (p)
= 0.987 bar
According
to Henry’s law:
p = KHx
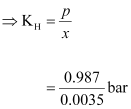
= 282
bar