NEET-XII-Chemistry
c2022 year:2022
- Qstn #15Match List - I with List - II.
Choose the correct answer from the options given below:List I(Products formed) List II (Reaction of carbonyl compound with a Cyanohydrin i `` NH_2OH `` b Acetal ii `` RNH_2 `` c Schiff's base iii alcohol d Oxime iv HCN
(A)
(a) - (iv),
(b) - (iii),
(c) - (ii),
(d) - (i)
(B)
(a) - (iii),
(b) - (iv),
(c) - (ii),
(d) - (i)
(C)
(a) - (ii),
(b) - (iii),
(c) - (iv),
(d) - (i)
(D)
(a) - (i),
(b) - (iii),
(c) - (ii),
(d) - (iv)digAnsr: AAns :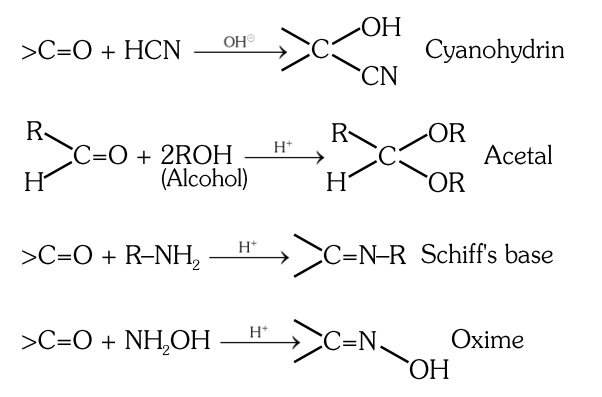
- Qstn #16Match List - I with List - II.
Choose the correct answer from the options given below:List I(Hydrides) List II (Nature) a `` MgH _{2} `` i Electron precise b `` GeH _{4} `` ii Electron deficient c `` B _{2} H _{6} `` iii Electron rich d `` HF `` iv Ionic
(A)
(a) - (ii),
(b) - (iii),
(c) - (iv),
(d) - (i)
(B)
(a) - (iv),
(b) - (i),
(c) - (ii),
(d) - (iii)
(C)
(a) - (iii),
(b) - (i),
(c) - (ii),
(d) - (iv)
(D)
(a) - (i),
(b) - (ii),
(c) - (iv),
(d) - (iii)digAnsr: BAns : Electron deficient hydride `` \rightarrow `` Less than `` 8 e^{-}\left( B _{2} H _{6}\right) ``
Electron precise hydride `` \rightarrow `` having `` 8 e^{-} `` without l.p. `` \left( GeH _{4}\right) ``
Electron rich hydride `` \rightarrow `` having `` 8 e^{-} `` with l.p. `` (HF) ``
- Qstn #17Gadolinium has a low value of third ionisation enthalpy because of
(A)high basic character
(B)small size
(C)high exchange enthalpy
(D)high electronegativitydigAnsr: CAns : `` { }_{64} Gd =[ Xe ] 6 s ^{2} 4 f ^{7} 5 d ^{1} ``
`` Gd ^{+2} =[ Xe ] 4 f ^{7} 5 d ^{1} ``
After losing `` 5 d `` electron `` 4 f `` has maximum exchange energy so Gd has low value of Third Ionisation energy
- Qstn #18Given below are two statements: Statement I: In the coagulation of a negative sol, the flocculating power of the three given ions is in the order - `` Al ^{3+}> Ba ^{2+}> Na ^{+} `` Statement II : In the coagulation of a positive sol, the flocculating power of the three given salts is in the order - `` NaCl > Na _{2} SO _{4}> Na _{3} PO _{4} `` In the light of the above statements, choose the most appropriate answer from the options given below :
(A)Statement I is incorrect but Statement II is correct.
(B)Both Statement I and Statement II are correct.
(C)Both Statement I and Statement II are incorrect.
(D)Statement I is correct but Statement II is incorrect.digAnsr: DAns : According to Hardy Schulze Rule statement 1 is correct. (Generally, the greater the valence of the flocculating ion added, the greater is its power to cause precipitation)
According to Hardy Schulze Rule statement 2 is incorrect
- Qstn #19Match List - I with List - II.
Choose the correct answer from the options given below:List I(Drug class) List II (Drug molecule) a Antacids i Salvarsan b Antihistamines ii Morphine c Analgesics iii Cimetidine d Antimicrobials iv Seldane
(A)
(a) - (iv),
(b) - (iii),
(c) - (i),
(d) - (ii)
(B)
(a) - (iii),
(b) - (ii),
(c) - (iv),
(d) - (i)
(C)
(a) - (iii),
(b) - (iv),
(c) - (ii),
(d) - (i)
(D)
(a) - (i),
(b) - (iv),
(c) - (ii),
(d) - (iii)digAnsr: AAns : Antacid - Cimetidine
Antihistamine - Seldane
Analgesic - Morphine
Antimicrobials - Salvarsan
- Qstn #20The Kjeldahl's method for the estimation of nitrogen can be used to estimate the amount of nitrogen in which one of the following compounds?
(A)

(B)

(C)

(D)
 digAnsr: DAns :
digAnsr: DAns :
Kjeldahl's method is not applicable to the compounds containing nitrogen having nitro and azo group and nitrogen present in the ring (pyridine), as nitrogen of these compounds does not change to ammonium sulphate under these conditions.
- Qstn #21Choose the correct statement:
(A)Both diamond and graphite are used as dry lubricants.
(B)Diamond and graphite have two dimensional network.
(C)Diamond is covalent and graphite is ionic.
(D)Diamond is `` s p^{3} `` hybridised and graphite is `` s p^{2} `` hybridized.digAnsr: DAns : In diamond each carbon is bonded with four other carbon atoms. So hybridisation of carbon atom is `` sp ^{3} `` .
In graphite each carbon is bonded with three other carbon atoms. So hybridisation of carbon atom is `` sp ^{2} `` .
- Qstn #22The IUPAC name of the complex - `` \left[ Ag \left( H _{2} O \right)_{2}\right]\left[ Ag ( CN )_{2}\right] `` is :
(A)diaquasilver(I) dicyanidoargentate(I)
(B)dicyanidosilver(II) diaquaargentate(II)
(C)diaquasilver(II) dicyanidoargentate(II)
(D)dicyanidosilver(I) diaquaargentate(I)digAnsr: CAns : IUPAC
`` \left[ Ag \left( H _{2} O \right)_{2}\right]\left[ Ag ( CN )_{2}\right] ``
Coordination number `` =2 `` ,
Oxidation state `` = Ag ^{+1} ``
Diaquasilver(I) dicyanidoargentate(I)
- Qstn #23Which of the following `` p - V `` curve represents maximum work done?
(A)

(B)

(C)

(D)
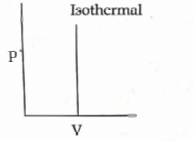 digAnsr: CAns : In `` P - V `` graph area under the curve represent magnitude of work.
digAnsr: CAns : In `` P - V `` graph area under the curve represent magnitude of work.
As it is maximum in graph- `` 1 ``
- Qstn #24In one molal solution that contains `` 0.5 `` mole of a solute, there is
(A) `` 1000\, g `` of solvent
(B) `` 500 \, mL `` of solvent
(C) `` 500\, g `` of solvent
(D) `` 100\, mL `` of solventdigAnsr: CAns : `` m =\frac{\text { Moles of solute }}{\text { Weight of solvent }( g )} \times 1000 ``
`` 1=\frac{0.5}{\text { Weight of solvent }( g )} \times 1000 ``
Weight of solvent `` ( g )=500\, g ``
- Qstn #25Identify the incorrect statement from the following.
(A)The shapes of `` d_{x y}, d_{y z} `` and `` d_{z x} `` orbitals are similar to each other; and `` d_{x^{2}-y^{2}} `` and `` d_{z} 2 `` are similar to each other.
(B)All the five `` 5 d `` orbitals are different in size when compared to the respéctive `` 4 d `` orbitals.
(C)All the five `` 4 d `` orbitals have shapes similar to the respective `` 3 d `` orbitals.
(D)In an atom, all the five `` 3 d `` orbitals are equal in energy in free state.digAnsr: AAns :
- Qstn #26What mass of `` 95 \% `` pure `` CaCO _{3} `` will be required to neutralise `` 50 \, mL `` of `` 0.5\, M HCl `` solution according to the following reaction? `` CaCO _{3( s )}+2 HCl _{( aq )} \rightarrow CaCl _{2( aq )}+ CO _{2( g )}+2 H _{2} O _{( l )} `` [Calculate upto second place of decimal point]
(A) `` 9.50\, g ``
(B) `` 1.25 \, g ``
(C) `` 1.32\, g ``
(D) `` 3.65 \, g ``digAnsr: CAns : `` CaCO _{3( s )}+2 HCl _{\text {(aq.) }} \rightarrow CaCl _{2 \text { (aq.) }}+ CO _{2( a )}+ H _{2} O _{(0)} ``
no. of moles of `` CaCO _{3} `` (pure) `` =\frac{1}{2} \times `` mole of `` HCl ``
`` \text { [Mole }=\text { molarity } \times \text { volume(in ltr. })] ``
`` =\frac{1}{2} \times 0.5 \times \frac{50}{1000}=0.0125 ``
weight of `` CaCO _{3} `` (pure) `` = `` mole `` \times `` mol. wt
`` =0.0125 \times 100=1.25\,g ``
`` \% `` purity `` =\frac{\text { wt. of pure substance }}{\text { wt. of impure sample }} \times 100 ``
`` 95=\frac{1.25}{\text { wt. of impure sample }} \times 100 ``
wt. of impure sample `` =\frac{1.25 \times 100}{95}=1.32\, g ``
- Qstn #27Which amongst the following is incorrect statement?
(A) `` O _{2}^{+} `` ion is diamagnetic.
(B)The bond orders of `` O _{2}^{+}, O _{2}, O _{2}^{-} `` and `` O _{2}^{2-} `` are `` 2.5,2,1.5 `` and `` 1 `` , respectively.
(C) `` C _{2} `` molecule has four electrons in its two degenerate `` \pi `` molecular orbitals.
(D) `` H _{2}^{+} `` ion has one electron.digAnsr: AAns : `` O _{2}^{+} `` ion is having `` 15 `` electrons, so it contain one unpaired electron. Hence it is paramagnetic in nature.
- Qstn #28`` RMgX + CO_2 \xrightarrow[\text{ether}]{\text{dry}} Y \xrightarrow{H_3O^+} RCOOH `` What is `` Y `` in the above reaction?
(A) `` R _{3} CO ^{-} Mg ^{+} X ``
(B) `` RCOO ^{-} X ^{+} ``
(C) `` ( RCOO )_{2} Mg ``
(D) `` RCOO ^{-} Mg ^{+} X ``digAnsr: DAns :
- Qstn #29Given below are half cell reactions: `` MnO _{4}^{-}+8 H ^{+}+5 e ^{-} \rightarrow Mn ^{2+}+4 H _{2} O `` `` E _{ Mn ^{2+} / MnO _{4}^{-}}^{\circ}=-1.510\, V `` `` \frac{1}{2} O _{2}+2 H ^{+}+2 e ^{-} \rightarrow H _{2} O , `` `` E _{ O _{2} / H _{2} O }^{\circ}=+1.223 \,V `` Will the permanganate ion, `` MnO _{4}^{-} `` liberate `` O _{2} `` from water in the presence of an acid ?
(A)No, because `` E _{\text {cell }}^{\circ}=-2.733 \,V ``
(B)Yes, because `` E _{\text {cell }}^{\circ}=+0.287\, V ``
(C)No, because `` E _{\text {cell }}^{\circ}=-0.287 \,V ``
(D)Yes, because `` E _{\text {cell }}^{\circ}=+2.733 \,V ``digAnsr: BAns :
