NEET-XII-Chemistry
Previous Year Paper year:2021
- Qstn #14\( BF _{3} \) is planar and electron deficient compound. Hybridization and number of electrons around the central atom, respectively are :
(A) \( sp ^{3} \) and 4
(B) \( sp ^{3} \) and 6
(C) \( sp ^{2} \) and 6
(D) \( sp ^{2} \) and 8digAnsr: CAns : C
- Qstn #15Which one among the following is the correct option for right relationship between \( C _{ P } \) and \( C _{ V } \) for one mole of ideal gas?
(A) \( C _{ P }+ C _{ V }= R \)
(B) \( C _{ P }- C _{ V }= R \)
(C) \( C _{ P }= RC _{ V } \)
(D) \( C _{ V }= RC _{ P } \)digAnsr: BAns : B
- Qstn #16Among the following alkaline earth metal halides, one which is covalent and soluble in organic solvents is :
(A) Calcium chloride
(B) Strontium chloride
(C) Magnesium chloride
(D) Beryllium chloridedigAnsr: DAns : D
- Qstn #17An organic compound contains \( 78 \% \) (by wt.) carbon and remaining percentage of hydrogen. The right option for the empirical formula of this compound is: [Atomic wt. of \( C \) is \( 12, H \) is \( 1 \) ]
(A) \( CH \)
(B) \( CH _{2} \)
(C) \( CH _{3} \)
(D) \( CH _{4} \)digAnsr: CAns : C
- Qstn #18The major product formed in dehydrohalogenation reaction of \( 2-Bromo \) pentane is \( Pent-2-ene \) . This product formation is based on ?
(A) Saytzeff's Rule
(B) Hund’s Rule
(C) Hofmann Rule
(D) Huckel’s RuledigAnsr: AAns : A
- Qstn #19What is the IUPAC name of the organic compound formed in the following chemical reaction ? \( \text{Acetone} \ce{->[(i)C_2H_5MgBr, \text{dry Ether}][(ii)H_2O, H^+]} \text{Product} \)
(A) 2-methyl propan-2-ol
(B) pentan-2-ol
(C) pentan-3-ol
(D) 2-methyl butan-2-oldigAnsr: DAns : D
- Qstn #20Noble gases are named because of their inertness towards reactivity. Identify an incorrect statement about them.
(A) Noble gases are sparingly soluble in water.
(B) Noble gases have very high melting and boiling points.
(C) Noble gases have weak dispersion forces.
(D) Noble gases have large positive values of electron gain enthalpy.digAnsr: BAns : B
- Qstn #21The \( pK _{ b } \) of dimethylamine and \( pK _{ a } \) of acetic acid are \( 3.27 \) and \( 4.77 \) respectively at \( T ( K ) \) . The correct option for the \( pH \) of dimethylammonium acetate solution is :
(A) \( 8.50 \)
(B) \( 5.50 \)
(C) \( 7.75 \)
(D) \( 6.25 \)digAnsr: CAns : C
- Qstn #22The right option for the statement ''Tyndall effect is exhibited by'', is :
(A) NaCl solution
(B) Glucose solution
(C) Starch solution
(D) Urea solutiondigAnsr: CAns : C
- Qstn #23Statement I : Acid strength increases in the order given as \( HF < < HCl < < HBr < < HI \) . Statement II : As the size of the elements \( F, Cl, Br, I \) increases down the group, the bond strength of \( HF, HCl, HBr \) and \( HI \) decreases and so the acid strength increases. In the light of the above statements, choose the correct answer from the options given below.
(A) Both Statement I and Statement II are true.
(B) Both Statement I and Statement II are false.
(C) Statement I is correct but Statement II is false.
(D) Statement I is incorrect but Statement II is true.digAnsr: AAns : A
- Qstn #24Ethylene diaminetetraacetate (EDTA) ion is :
(A) Hexadentate ligand with four ''O'' and two ''N'' donor atoms
(B) Unidentate ligand
(C) Bidentate ligand with two ''N'' donor atoms
(D) Tridentate ligand with three ''N'' donor atomsdigAnsr: AAns : A
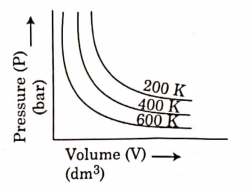
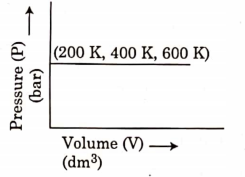
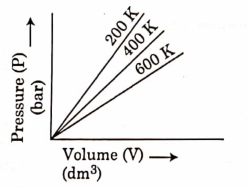
- Qstn #25Choose the correct option for graphical representation of Boyle’s law, which shows a graph of pressure vs. volume of a gas at different temperatures:
(A)
(B)
(C)
(D)
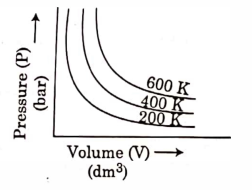 digAnsr: CAns : C
digAnsr: CAns : C
- Qstn #26The structures of beryllium chloride in solid state and vapour phase, are :
(A) Chain and dimer, respectively
(B) Linear in both
(C) Dimer and Linear, respectively
(D) Chain in bothdigAnsr: DAns : D
- Qstn #27Which one of the following methods can be used to obtain highly pure metal which is liquid at room temperature ?
(A) Electrolysis
(B) Chromatography
(C) Distillation
(D) Zone refiningdigAnsr: CAns : C
- Qstn #28The compound which shows metamerism is :
(A) \( C _{5} H _{12} \)
(B) \( C _{3} H _{8} O \)
(C) \( C _{3} H _{6} O \)
(D) \( C _{4} H _{10} O \)digAnsr: DAns : D