NEET-XII-Chemistry
exam-1 year:2016
- Qstn #75The pressure of ``H_2`` required to make the potential
of ``H_2``-electrode zero in pure water at 298 K is :-
(1) ``10^{-14}`` atm
(2) ``10^{-12}`` atm
(3) ``10^{-10}`` atm
(4) ``10^{-4}`` atmdigAnsr: 1Ans : (1)
Sol. 2H+(aq) + 2e- H2(g)
+
∴ = 2
H0
2
P0.0591
E E log
2 H
0 = 0- 0.0295 log
2H
27
P
10
2H
27
P
10
=1
=
2
14
HP 10 atm
- Qstn #76The addition of a catalyst during a chemical reaction
alters which of the following quantities ?
(1) Entropy
(2) Internal energy
(3) Enthalpy
(4) Activation energydigAnsr: 4Ans : (4)
Sol. The addition of catalyst during a chemical reaction
alters the activation energy.
- Qstn #77The ionic radii of ``A^+`` and ``B^-`` ions are
0.98 × ``10^{-10}`` m and 1.81 × ``10^{-10}`` m. The
coordination number of each ion in AB is :-
(1) 6
(2) 4
(3) 8
(4) 2digAnsr: 1Ans : (1)
Sol. radii ratio =
+
= =
10
10
r 0.98 10
0.54
r 1.81 10
radii ratio is in between 0.414 to 0.732
so, coordination number is 6
- Qstn #78Which is the correct statement for the given acids?
(1) Phosphinic acid is a diprotic acid while
phosphonic acid is a monoprotic acid
(2) Phosphinic acid is a monoprotic acid while
phosphonic acid is a diprotic acid
(3) Both are triprotic acids
(4) Both are diprotic acidsdigAnsr: 2Ans : (2)
Sol. Phosphinic acid (H3PO2)
P
H
OHH
O
Monoprotic
Phosphonic acid (H3PO3)
P
OH OHH
O
Diprotic acid
- Qstn #79Fog is colloidal solution of :-
(1) Liquid in gas
(2) Gas in liquid
(3) Solid in gas
(4) Gas in gasdigAnsr: 1Ans : (1)
Sol. Fog is a colloidal solution of liquid in gas
- Qstn #80Which of the following statement about the
composition of the vapour over an ideal a 1 : 1 molar
mixture of benzene and toluene is correct? Assume
that the temperature is constant at 25°C.
(Given : Vapour Pressure Data at 25°C,
benzene = 12.8 kPa, Toluene = 3.85 kPa)
(1) The vapour will contain a higher percentage of
benzene
(2) The vapour will contain a higher percentage of
toluene
(3) The vapour will contain equal amounts of
benezene and toluene
(4) Not enough information is given to make a
predicationdigAnsr: 1Ans : (1)
Sol. A benzene, B toluene
1 : 1 molar mixture of A and B
∴ =A
1
x
2
and =B
1
x
2
= +0 0s A A B BP P X P X
= + =s
1 1
P 12.8 3.85 8.325kPa
2 2
= = =
0
A A
A
s
1
12.8P X 2Y 0.768
P 8.325
∴ = = =B AY 1 Y 1 0.768 0.232
so, the vapour will contain higher percentage of
benzene.
- Qstn #81The correct statement regarding the comparison
of staggered and eclipsed conformation of ethane,
is :-
(1) The staggered conformation of ethane is less
stable than eclipsed conformation, because
staggered conformation has torsional strain
(2) The eclipsed conformation of ethane is more
stable than staggered conformation, because
eclipsed conformation has no torsional strain
(3) The eclipsed conformation of ethane is more
stable than staggered conformation even
through the eclipsed conformation has torsional
strain
(4) The staggered conformation of ethane is more
stable than eclipsed conformation, because
staggered conformation has no torsional strain.digAnsr: 4Ans : (4)
Sol.
H H
H
H
H H
H H
H
H H
H
Staggered form
* No torsional strain
Eclipsed form
- Qstn #82The reaction
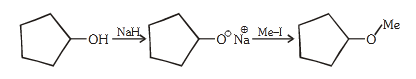
Can be classified as :-
(1) Williamson ether synthesis reaction
(2) Alcohol formation reaction
(3) Dehydration reaction
(4) Williamson alcohol synthesis reactiondigAnsr: 1Ans : (1)
Sol. This is an exmaple of Williamson ether syntehsis
reaction in which sodium alkoxide reacts with alkyl
halide and gives ether.
- Qstn #83The product formed by the reaction of an aldehyde
with a primary amine is :-
(1) Schiff base
(2) Ketone
(3) Carboxylic acid
(4) Aromatic aciddigAnsr: 1Ans : (1)
Sol.
C O
R
H
H
+
C N
R
H
R'
Aldehyde
+ R'—NH2
+ primary amine Schiff base
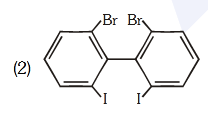
- Qstn #84Which of the following biphenyls is optically active?
(1)
(2)
(3)
(4)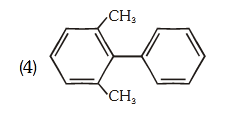 digAnsr: 2Ans : (2)
digAnsr: 2Ans : (2)
Sol.
Br
I
Br
I
is optically active due to
absence of plane of symmetry and center of
symmetry
- Qstn #85For the following reactions :-
a) ``CH_3CH_2CH_2Br + KOH \rightarrow CH_3CH=CH_2+KBr + H_2O``
(b)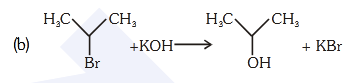
(c)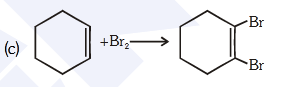
Which of the following statements is correct ?
(1) (a) and (b) are elimination reaction and (c) is
addition reaction
(2) (a) is elimination, (b) is substitution and (c) is
addition reaction
(3) (a) is elimination, (b) and (c) are substitution
reactions
(4) (a) is substitution, (b) and (c) are addition reactiondigAnsr: 2Ans : (2)
Sol.
(a) CH3CH2CH2-Br+KOH CH3CH=CH2+KBr + H2O
breaking of 2 bonds and formation of 1 bond so
it is an example of elimination reaction.
(b)
C
Br
CH3CH3 C
OH
CH3CH3
+ KOH + KBr
replacement of Br- by OH- is substitution reaction
(c) + Br2
Br
Br
breaking of 1 bond and formation of 2 bonds is
addition reaction
- Qstn #86At 100°C the vapour pressure of a solution
of 6.5g of a solute in 100 g water is 732 mm.
If ``K_b`` = 0.52,
the boiling point of this solution will be :-
(1) 101°C
(2) 100°C
(3) 102°C
(4) 103°CdigAnsr: 1Ans : (1)
Sol.
0
s solute solvent
0
solute solvent
P P w Mn
N M WP
= =
at 100 °C, P0 = 760 mm
=
solute
760 732 6.5 18
760 M 100
Msolute = 31.75 g mol
-1
▵Tb = m× Kb =
solute
solute solvent
w 1000
M w × Kb
▵ = =
b
0.52 6.5 1000
T 1.06 C
31.75 100
∴ boiling point of solution
= 100°C + 1.06°C 101°C
- Qstn #87The correct statement regarding RNA and DNA,
respectively is :
(1) The sugar component in RNA is arabinose and
the sugar component in DNA is 2'-deoxyribose.
(2) The sugar component in RNA is ribose and the
sugar component in DNA is 2'-deoxyribose.
(3) The sugar component in RNA is arabinose
(4) The sugar component in RNA is 2'-deoxyribose
and the sugar component in DNA is arabinose.digAnsr: 2Ans : (2)
Sol. RNA Ribose Nucleic Acid
DNA 2'-Deoxyribose Nucleic Acid
- Qstn #88The correct statement regarding the basicity
of arylamines is :-
(1) Arylamines are generally less basic than
alkylamines because the nitrogen lone-pair
electrons are delocalized by interaction with the
aromatic ring ``\pi`` electron system.
(2) Arylamines are generally more basic than
alkylamines because the nitrogen lone-pair
electrons are not delocalized by interaction with
the aromatic ring ``\pi``electron system.
(3) Arylamines are generally more basic than
alkylamines because of aryl group.
(4) Arylamines are generally more basic than
alkylamines, because the nitrongen atom in
arylamines is sp-hybridized.digAnsr: 1Ans : (1)
Sol.
NH2
..
Aryl amine
..
R NH2
alkyl amine
* Delocalized lone pair of nitrogen
* less basic
- Qstn #89Which one given below is a non-reducing sugar ?
(1) Maltose
(2) Lactose
(3) Glucose
(4) SucrosedigAnsr: 4Ans : (4)
Sol.
NH2
..
Aryl amine
..
R NH2
alkyl amine
* Delocalized lone pair of nitrogen
* less basic