NEET-XII-Chemistry
exam-1 year:2016
- Qstn #46Consider the molecules ``CH_4, NH_3 and H_2O``.
Which of the given statements is false ?
(1) The H-C-H bond angle in ``CH_4``, the H-N-H
bond angle in ``NH_3``, and the H-O-H bond angle
in ``H_2O`` are all greater than 90°
(2) The H-O-H bond angle in ``H_2O`` is larger than
the H-C-H bond angle in ``CH_4``.
(3) The H-O-H bond angle in ``H_2O`` is smaller than
the H-N-H bond angle in ``NH_3``.
(4) The H-C-H bond angle in ``CH_4`` is larger than
the H-N-H bond angle in ``NH_3``.digAnsr: 2Ans : (2)
Sol.
C
H
HH
H
109°28'
N
H
HH
..
107°
O
H H
.. 104.5°
..
CH4 NH3 H O2
- Qstn #47In the reaction
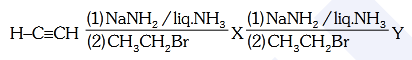
X and Y are :
(1) X = 1-Butyne ; Y = 3-Hexyne
(2) X = 2-Butyne ; Y = 3-Hexyne
(3) X = 2-Butyne ; Y = 2-Hexyne
(4) X = 1-Butyne ; Y = 2-HexynedigAnsr: 1Ans : (1)
Sol. CH CH NaNH2liq. NH3 CH CNa
CH3 CH2 Br CH C CH2 CH3
(X)
CH3 CH2 C C CH2 CH3
CH3 CH2 Br CH3 CH2 C CNa
NaNH2 liq. NH3
1-Butyne
3-Hexyne
- Qstn #48Among the following, the correct order of acidity is
(1) ``\ce { HClO3 < HClO4 < HClO2 < HClO} ``
(2) ``\ce { HClO < HClO2 < HClO3 < HClO4} ``
(3) ``\ce { HClO2 < HClO < HClO3 < HClO4} ``
(4) ``\ce { HClO4 < HClO2 < HClO < HClO3} ``digAnsr: 2Ans : (2)
Sol. Acidic strength EN +ve O.S.
HClO < HClO2 < HClO3 < HClO4
+1 +3 +5 +7
- Qstn #49The rate of a f irst -order react ion is
0.04 mol ``l^{-1} s^{-1}`` at 10 seconds and
0.03 mol ``l^{-1} s^{-1}``at 20 seconds after initiation of the
reaction. The half-life period of the reaction is :
(1) 24.1 s
(2) 34.1 s
(3) 44.1 s
(4) 54.1 sdigAnsr: 1Ans : (1)
Sol.
1
2 1 2
a x2.303
K log
(t t ) a x
=
2.303 0.04
K log
20 10 0.03
=
2.303 0.1249
K
10
=
=
1/2
2.303 log2 2.303 0.1249
t 10
= =1/2
0.3010 10
t 24.1 sec
0.1249
- Qstn #50Which one of the following characteristics is
associated with adsorption ?
(1) ``\triangle``G is negative but ``\triangle``H and ``\triangle``S are positive
(2) ``\triangle``G, ``\triangle``H and ``\triangle``S all are negative
(3) ``\triangle``G and ``\triangle``H are negative but ``\triangle``S is positive
(4) ``\triangle``G and ``\triangle``S are negative but ``\triangle``H is positivedigAnsr: 2Ans : (2)
Sol. Adsorption is spontaneous process,
so ▵G = negative
Adsorption is exothermic process,
so ▵H = negative
In adsorpiton entropy decreases,
so ▵S = negative
so ▵G, ▵H and ▵S all are negative
- Qstn #51In which of the following options the order of
arrangement does not agree with the variation of
property indicated against it ?
(1) ``\ce {Al^3+ < Mg^2+ < Na+ < F- } ``(increasing ionic size)
(2) B < C < N < O (increasing first ionisation enthalpy)
(3) I < Br < Cl < F (increasing electron gain
enthalpy)
(4) Li < Na < K < Rb (increasing metallic radius)digAnsr: 2Ans : (2 & 3)
Sol. (2) B < C < N < O (given I.P. order)
B < C < O < N (correct)
(3) I < Br < Cl < F (given ▵Heg order)
I < Br < F < Cl (Correct)
- Qstn #52Which of the following statements is false ?
(1) ``Mg^{2+}`` ions form a complex with ATP
(2) ``Ca^{2+}`` ions are important in blood clotting
(3) ``Ca^{2+}`` ions are not important in maintaining the
regular beating of the heart.
(4)``Mg^{2+}`` ions are important in the green parts of
plants.digAnsr: 3Ans : (3)
Sol.
- Qstn #53Which of the following statements about hydrogen
is incorrect ?
(1) hydrogen has three isotopes of which tritium is
the most common.
(2) Hydrogen never acts as cation in ionic salts
(3) Hydronium ion, ``H_3O^+`` exists freely in solution
(4) Dihydrogen does not act as a reducing agentdigAnsr: 1Ans : (1 & 4)
Sol.
- Qstn #54The correct statement regarding a carbonyl
compound with a hydrogen atom on its
alphacarbon,is :-
(1) a carbonyl compound with a hydrogen atom on
its alpha-carbon never equilibrates with its
corresponding enol.
(2) a carbonyl compound with a hydrgen atom on
its alpha-carbon rapidly equilibrates with its
corresponding enol and this process is known
as aldehyde-ketone equilibration.
(3) a carbonyl compound with a hydrogen atom on
its alpha-carbon rapidly equilibrates with its
corresponding enol and this process is known
as carbonylation.
(4) a carbonyl compound with a hydrogen atom on
its alpha-carbon rapidly equilibrates with its
corresponding enol and this process is known
as keto-enol tautomerism.digAnsr: 4Ans : (4)
Sol. Keto-enol Tautomerism
C
H
C
O
C C
OH
(enol)
(Keto)
- Qstn #55MY and ``NY_3``, two nearly insoluble salts, have the
same ``K_{sp}`` values of 6.2 × ``10^{-13}`` at room
temperature. Which statement would be true in
regard to MY and ``NY_3`` ?
(1) The molar solubilities of MY and ``NY_3`` in water
are identical.
(2) The molar solubility of MY in water is less than
that of ``NY_3``
(3) The salts MY and ``NY_3`` are more soluble in
0.5 M KY than in pure water.
(4) The addition of the salt of KY to solution of MY
and ``NY_3`` will have no effect on their solubilities.digAnsr: 2Ans : (2)
Sol. = 2spMY K s = 6.2 × 10-13
= 13s 6.2 10
s = 7.87 × 10-7 mol L-1
NY3 Ksp = 27 s
4 = 6.2 × 10-13
=
1/4136.2 10
s
27
s = 3.89 × 10-4 mol L-1
∴ molar solubility of NY3 is more than MY in wa-
ter.
- Qstn #56In a protein molecule various amino acids are linked
together by :
(1) ``\alpha``-glycosidic bond
(2) ``\beta``-glycosidic bond
(3) peptide bond
(4) dative bonddigAnsr: 3Ans : (3)
Sol. Peptide bond
C
O
NH
- Qstn #57Natural rubber has
(1) All cis-configuration
(2) All trans-configuration
(3) Alternate cis-and trans-configuration
(4) Random cis-and trans-configurationdigAnsr: 1Ans : (1)
Sol. CH2 C CH CH2
CH3
Isoprene
polymerisation CH2
CH3 H
CH2
n
cis-polyisoprene
- Qstn #58Match items of Column I with the items of
Column II and asign the correct code :
Column-I Column-II
a) Cyanide (i) Ultrapure Ge
process
(b)Froth floatation (ii) Dressing of ZnS
process
(c)Electrolytic (iii) Extraction of Al
reduction
(d) Zone refining (iv) Extraction of Au
(v) Purification of Ni
Code :
a) (b) (c) (d)
(1) (iv) (ii) (iii) (i)
(2) (ii) (iii) (i) (v)
(3) (i) (ii) (iii) (iv)
(4) (iii) (iv) (v) (i)digAnsr: 1Ans : (1)
Sol.
- Qstn #59Which one of the following statements is correct
when ``SO_2`` is passed through acidified ``K_2Cr_2O_7``
solution ?
(1) The solution turns blue
(2) The solution is decolourized
(3) ``SO_2`` is reduced
(4) Green ``Cr_2(SO_4)_3`` is formeddigAnsr: 4Ans : (4)
Sol. K2Cr2O7 + SO2 + H2SO4
K2SO4 + Cr2(SO4)3 + H2O
green colour