NEET-XII-Chemistry
Previous Year Paper year:2020
- Qstn #120Which of the following is not correct about carbon
monoxide ?
(1) It forms carboxyhaemoglobin.
(2) It reduces oxygen carrying ability of blood.
(3) The carboxyhaemoglobin (haemoglobin
bound to CO) is less stable than
oxyhaemoglobin.
(4) It is produced due to incomplete combustion.digAnsr: 3Ans : 3
- Qstn #121Sucrose on hydrolysis gives :
(1) β-D-Glucose+α-D-Fructose
(2) α-D-Glucose+β-D-Glucose
(3) α-D-Glucose+β-D-Fructose
(4) α-D-Fructose+β-D-FructosedigAnsr: 3Ans : 3
- Qstn #122The following metal ion activates many enzymes,
participates in the oxidation of glucose to produce
ATP and with Na, is responsible for the
transmission of nerve signals.
(1) Iron
(2) Copper
(3) Calcium
(4) PotassiumdigAnsr: 4Ans : 4
- Qstn #123Which one of the followings has maximum number
of atoms ?
(1) 1 g of Ag(s) [Atomic mass of Ag=``10^{8}``]
(2) 1 g of Mg(s) [Atomic mass of Mg=24]
(3) 1 g of ``\ce{O2}``
(g) [Atomic mass of O=16]
(4) 1 g of Li(s) [Atomic mass of Li=7]digAnsr: 4Ans : 4
- Qstn #124The number of protons, neutrons and electrons in
``\ce{ ^{175}_{71}Lu+ }``, respectively, are :
(1) 71, 104 and 71
(2) 104, 71 and 71
(3) 71, 71 and 104
(4) 175, 104 and 71digAnsr: 1Ans : 1
- Qstn #125What is the change in oxidation number of carbon
in the following reaction ?
``\ce{CH4(g) + 4Cl2(g) -> CCl4(l) + 4HCl(g)}``
(1) +4 to +4
(2) 0 to +4
(3) -4 to +4
(4) 0 to -4digAnsr: 3Ans : 3
- Qstn #126Identify the incorrect statement.
(1) ``\ce{Cr^{2+}(d^4)}`` is a stronger reducing agent than
``\ce{Fe2+(d^6)}`` in water.
(2) The transition metals and their compounds
are known for their catalytic activity due to
their ability to adopt multiple oxidation
states and to form complexes.
(3) Interstitial compounds are those that are
formed when small atoms like H, C or N
are trapped inside the crystal lattices of
metals.
(4) The oxidation states of chromium in
``\ce{CrO4^{2-} }`` and ``\ce{Cr2O7^{2-} }``
are not the same.digAnsr: 4Ans : 4
- Qstn #127For the reaction,
``\ce{2Cl(g) -> Cl2(g)}``
, the correct option is :
(1) ``∆_rH > 0 and ∆_rS > 0``
(2) ``∆_rH > 0 and ∆_rS < 0``
(3) ``∆_rH < 0 and ∆_rS > 0``
(4) ``∆_rH < 0 and ∆_rS < 0``digAnsr: 4Ans : 4
- Qstn #128Measuring Zeta potential is useful in determining
which property of colloidal solution ?
(1) Viscosity
(2) Solubility
(3) Stability of the colloidal particles
(4) Size of the colloidal particlesdigAnsr: 3Ans : 3
- Qstn #129Urea reacts with water to form A which will
decompose to form B. B when passed through
``\ce{Cu^{2+}(aq)}``, deep blue colour solution C is formed.
What is the formula of C from the following ?
(1) ``\ce{CuSO4}``
(2) ``\ce{[Cu(NH3)4]^{2+}}``
(3) ``\ce{Cu(OH)2}``
(4) ``\ce{CuCO3.Cu(OH)2}``digAnsr: 2Ans : 2
- Qstn #130Match the following and identify the correct
option.
-(a) ``\ce{CO(g)+H2(g)}`` (i) ``\ce{Mg(HCO3)2+}`` ``\ce{Ca(HCO3)2}``
(b) Temporary hardness of water (ii) An electron deficient hydride
(c) ``\ce{B2H6 }`` (iii) Synthesis gas
(d) ``\ce{H2O2}`` (iv) Non-planar structure
-(a) (b) (c) (d)
(1) (iii) (i) (ii) (iv)
(2) (iii) (ii) (i) (iv)
(3) (iii) (iv) (ii) (i)
(4) (i) (iii) (ii) (iv)digAnsr: 1Ans : 1
- Qstn #131Match the following :
Oxide Nature
-(a) CO (i) Basic
(b) BaO (ii) Neutral
(c) ``\ce{Al2O3}`` (iii) Acidic
(d) ``\ce{Cl2O7}`` (iv) Amphoteric
Which of the following is correct option ?
-(a) (b) (c) (d)
(1) (i) (ii) (iii) (iv)
(2) (ii) (i) (iv) (iii)
(3) (iii) (iv) (i) (ii)
(4) (iv) (iii) (ii) (i)digAnsr: 2Ans : 2
- Qstn #132The rate constant for a first order reaction is
``4.606 \times 10^{-3}`` s-1. The time required to reduce
2.0 g of the reactant to 0.2 g is :
(1) 100 s
(2) 200 s
(3) 500 s
(4) 1000 sdigAnsr: 3Ans : 3
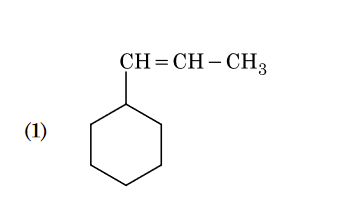
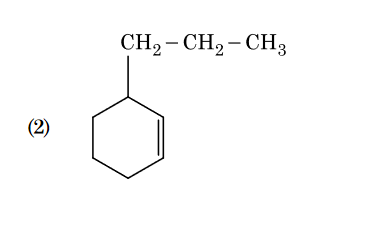
- Qstn #133An alkene on ozonolysis gives methanal as one of
the product. Its structure is :
(1)
(2)
(3)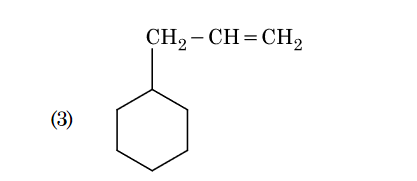
(4) digAnsr: 3Ans : 3
digAnsr: 3Ans : 3
- Qstn #134Which of the following alkane cannot be made in
good yield by Wurtz reaction ?
(1) n-Hexane
(2) 2,3-Dimethylbutane
(3) n-Heptane
(4) n-Butane17digAnsr: 3Ans : 3