ICSE-X-Physics
Previous Year Paper year:2019
- #8-c-iiithe resistance of the circuit when the key k is closed.
- #8-c-ivthe current drawn from thexell when the key k is closed.
- #9
- #9-a
- #9-a-iDefine Calorimetry.Ans : Calorimetry is the measurement of heat,
- #9-a-iiName the material used for making a Calorimeter.Ans : Copper.
- #9-a-iiiWhy is a Calorimeter made-up of thin sheets of the above material answered in (ii) ?
[3]
Ans : Copper has small specific heat capacity. The thin sheets ensure that the box has small heat capacity.
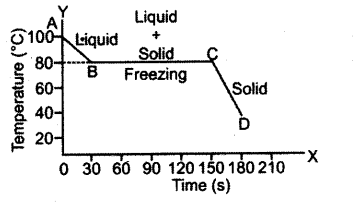
(b) The graph is as shown :
(c) Mass of water m1 = 104 g
Temperature of water T1 = 30°C
Final temperature T2 = 10°C
Mass of calorimeter m2 = 42 g
Temperature of ice T3= 0°C
Let the mass of ice added be = x g
Total heat energy lost = (104 × 4.2 × 20)+(42 × 0.4 × 20) = 8736 + 336 = 9072 J
Total heat energy gained = (336 × x)+(x × 4.2 × 10) = 336x + 42x = 378x
By principle of calorimetry,
Heat lost = Heat gained
378x =9072
x = ``\frac { 9072 }{ 378 }`` = 24g
- #9-bThe melting point of naphthalene is 80°C and the room temperature is 30°C. A sample of
liquid naphthalene at 100°C is cooled down to the room temperature. Draw a temperature¬time graph to represent this cooling. In the graph, mark the region which corresponds to the freezing process.
[3]
- #9-c104 g of water at 30°C is taken in a calorimeter made of copper of mass 42 g. When a certain mass of ice at 0°C is added to it, the final steady temperature of the mixture after the ice has melted, was found to be 10°C. Find the mass of ice added.
[Specific heat capacity of water = 4.2 J g-1 °C-1 : Specific latent heat of fusion of ice - 336 J g-1 ; Specific heat capacity of copper = 0.4 J g-1 °C-1 ]
[4]
- #10
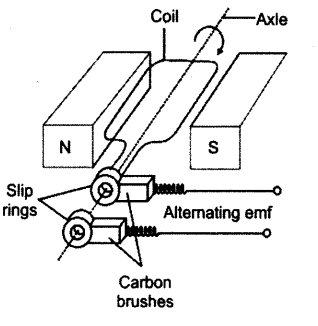
- #10-aDraw a neat labelled diagram of an AC generator.Ans : The diagram is as shown:
- #10-b
- #10-b-iDefine nuclear fission.Ans : It is the splitting of a heavy nudeus into two or more smaller nudei with the
release of tremendous energy.
- #10-b-iiRewrite and complete the following nuclear reaction by filling in the atomic number of Ba and mass number of Kr :
[3]
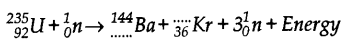
Ans :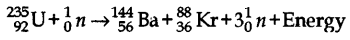
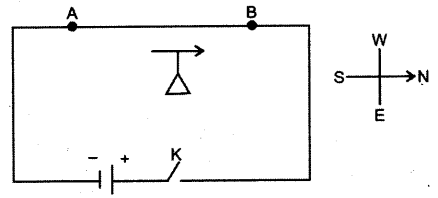
- #10-cThe diagram below shows a magnetic needle kept just below the conductor AB which is kept in North South direction.