ICSE-X-Chemistry
Previous Year Paper year:2010
- #6-a-iiiiii. Potassium sulphate from potassium hydroxide solution
- #6-a-iviv. Lead chloride from lead carbonate (two equations)
- #6-b [5]Compound A is bubbled through bromine dissolved in carbon tetrachloride and the
product is ``\ce{CH2Br.CH2Br}``.
$$\ce{A ->[\ce{Br2} ,\ce{CCl4}] CH2Br.CH2Br }$$
- #6-b-ii. Draw the structural formula of A.
- #6-b-iiii. What type of reaction has A undergone?
- #6-b-iiiiii. What is your observation?
- #6-b-iviv. Name (not formula) the compound formed when steam reacts with A in the
presence of phosphoric acid.
- #6-b-vv. What is the procedure for converting the product of (b) (iv) back to A?
- #7
- #7-a [3]The diagram shows a simple arrangement of the fountain experiment.
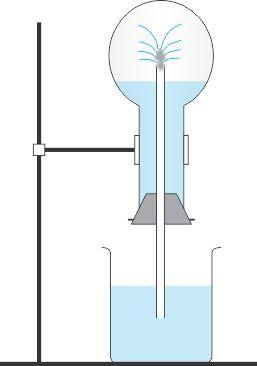
- #7-a-ii. Name the two gases you have studied which can be used in this experiment.
- #7-a-iiii. What is the common property demonstrated by this experiment?
- #7-b [2]Define the following terms:
- #7-b-ii. Ionisation potential
- #7-b-iiii. Electron affinity