ICSE-X-Chemistry
Previous Year Paper year:2010
- #4
- #4-a4.5 moles of calcium carbonate are reacted with dilute hydrochloric acid.
- #4-a-i [5]i. Write the equation for the reaction.
- #4-a-iiii. What is the mass of 4.5 moles of calcium carbonate? (Relative molecular mass of
calcium carbonate formed is 100.)
- #4-a-iiiiii. What is the volume of calcium dioxide liberated at STP?
- #4-a-iviv. What mass of calcium chloride is formed? (Relative molecular mass of calcium
chloride is 111.)
- #4-a-vv. How many moles of HCl are used in this reaction?
- #4-b [5]The diagram shows an apparatus for the laboratory preparation of hydrogen chloride.
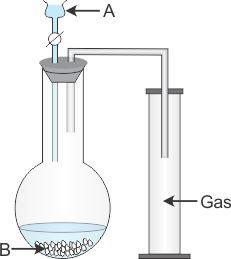
- #4-b-ii. Identify A and B.
- #4-b-iiii. Write the equation for the reaction.
- #4-b-iiiiii. How would you check whether the gas jar is filled with hydrogen chloride?
- #4-b-iviv. What does the method of collection tell you about the density of hydrogen chloride?
- #5
- #5-a [3]Name the main constituent metal in the following alloys:
- #5-a-ii. Duralumin