ICSE-X-Chemistry
Previous Year Paper year:2010
- #1-e-ii. Correct item from B matching sodium chloride
- #1-e-iiii. Correct item from B matching ammonium ion and so on.
- #1-f [5]Write the equation for each of the following reactions:
- #1-f-ii. Sulphur is heated with concentrated sulphuric acid.
- #1-f-iiii. Zinc oxide is treated with sodium hydroxide solution.
- #1-f-iiiiii. Ammonium chloride is heated with sodium hydroxide.
- #1-f-iviv. Concentrated sulphuric acid is poured over sugar.
- #1-f-vv. Magnesium sulphate solution is mixed with barium chloride solution.
- #1-g
- #1-g-i [5]i. LPG stands for liquefied petroleum gas. Varieties of LPG are marketed including a
mixture of propane (60%) and butane (40%). If 10 litre of this mixture is burnt,
then find the total volume of carbon dioxide gas added to the atmosphere.
Combustion reactions can be represented as
$$\ce{C3H8 (g) + 5 O2 (g) -> 3CO2 (g) + 4H2O (g) }$$
$$\ce{C4H10 (g) + 13 O2 (g) -> 8CO2 (g) + 10 H2O (g) }$$
- #1-g-iiii. Calculate the percentage of nitrogen and oxygen in ammonium nitrate (Relative
molecular mass of ammonium nitrate is 80, H = 1, N = 14, O = 16).
- # [40]Section : IIAttempt any four questions from this section.
- #2
- #2-a [5]Give the equations for the following conversions A to E.
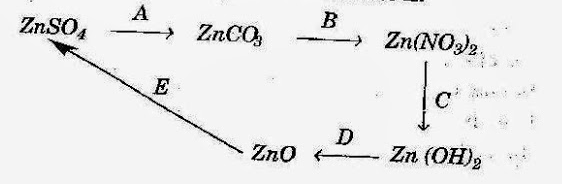
- #2-b [5]The questions below are related to the manufacture of ammonia.