ICSE-X-Chemistry
Previous Year Paper year:2017
- #3Ans : 3.
- #3-a [4]Draw an electron dot diagram to show the formation of each of the following
compounds:
i. Methane
ii. Magnesium Chloride
[H = 1, C = 6, Mg = 12, Cl = 17]Ans :
(i)
One atom of carbon shares four electron pairs, one with each of the four
atoms of hydrogen.
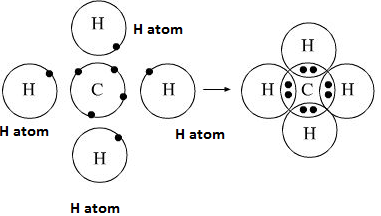
(ii)
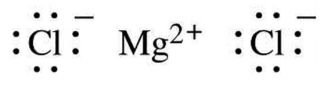
Here magnesium donates one electron each with two chlorine atoms
resulting in the formation of magnesium chloride.
- #3-b [4]State the observations at the anode and at the cathode during the electrolysis of:
i. fused lead bromide using graphite electrodes.
ii. copper sulphate solution using copper electrodes.Ans :
(i) Observations:
Anode: Dark reddish brown fumes of bromine evolve at the anode.
Cathode: Greyish white metal lead is formed on the cathode.
(ii) Observations:
Anode: Nothing gets deposited on the anode because the copper anode
dissolves during the reaction as Cu2+ ions are formed.
Cathode: Reddish brown Cu is deposited.
- #3-c [2]Select the ion in each case, that would get selectively discharged from the aqueous
mixture of the ions listed below:
i. ``\ce{{SO4}2-, {NO3}- and OH-}``
ii. ``\ce{Pb2+, Ag+ and Cu2+}``Ans :
(i) ``OH_-``
(ii) ``Ag^+``
- #4Ans : 4.
- #4-a [5]
Certain blank spaces are left in the following table and these are labelled as A, B, C, D and E. Identify each of them:
# Lab preparation of Reactants used Products formed Drying Agent Method of
collectioni ``\ce{HCl \text{gas}}`` ``\ce{NaCl + H2SO4}`` ___A___ ``\ce{conc. H2SO4}`` ____B___
II ``\ce{NH3}`` gas ____C___ ``\ce{Mg(OH)2NH3}`` ___D___ ___E___ Ans :
(i) ``\ce{A = NaHSO4 + HCl}``
B = upward displacement of air
(ii) ``\ce{C = Mg3N2 + H2O}``
D = Quicklime
E = downward displacement of air
- #4-b [3]Write balanced chemical equations to show:
i. The oxidizing action of conc. Sulphuric acid on carbon.
ii. The behavior of ``\ce{H2SO4}`` as an acid when it reacts with Magnesium.
iii. The dehydrating property of conc. Sulphuric acid with sugar.Ans :
(i)
``\ce{C + 2H2SO4 -> CO2 + 2H2O + 2SO2(^)}``
(ii)
``\ce{Mg + H2SO4 -> MgSO4 + H2(^)}``
(iii)
``\ce{C12H22O11 ->[][H2SO4] 12C + 11H2O}``
- #4-c [2]Write balanced chemical equations to show how ``\ce{SO3}`` is converted to Sulphuric acid in the contact process.Ans : ``\ce{SO3 + H2SO4 -> H2S2O7 }`` oleum or pyrosulphuric acid
``\ce{H2S2O7 + H2O -> 2H2SO4 }``
- #5Ans : 5.
- #5-a [4]
(i) Propane burns in air according to the following equation:
$$\ce{C3H8 + 5O2 -> 3CO2 + 4H2O}$$.
What volume of propane is consumed on using ``\pu{1000 cm3}`` of air, considering only 20% of air contains oxygen?Ans :
(i)
Given:
``\ce{C3H8 + 5O2 -> 3CO2 + 4H2O}``
Volume of air ``= 1000 cm^3``
Percentage of oxygen in air = 20%
According to Gay-Lussac.s law,
1 vol. of propane will consume 5 vol. of oxygen.
Volume of oxygen ``= 1000 cm^3 \times 20% = 200 cm^3``
Therefore,Volume of propane burnt for every ``200 cm^3`` of oxygen,
``\frac{1}{5}200= 40cm^3``
- #5-a-iiThe mass of 11.2 litres of a certain gas at s.t.p. is 24 g. Find the gram molecular mass of the gas.Ans : Given:
Volume of gas at STP = 11.2 litres
Mass of gas at STP = 24 g
Gram molecular mass = ?
The mass of 22.4 L of a gas at STP is equal to its gram molecular mass.
11.2 L of the gas at STP weighs 24 g
Therefore,
22.4 L of the gas will weigh
24
``\frac{24}{22.4} \times 11.2 = 48g ``
Gram molecular mass = 48 g
- #5-b [4]A gas cylinder can hold 1 kg of hydrogen at room temperature and pressure
i. Find the number of moles of hydrogen present.
ii. What weight of ``\ce{CO2}`` can the cylinder hold under similar conditions of temperature and pressure? (H = 1, C = 12, O = 16)
iii. If the number of molecules of hydrogen in the cylinder is X, calculate the number
of ``\ce{CO2}`` molecules in the cylinder under the same conditions of temperature and pressure.
iv. State the law that helped you to arrive at the above result.Ans : Given:
Mass of hydrogen = 1 kg at 298 K and 1 atm pressure
(i) Moles of hydrogen =?
Number of moles of hydrogen= Massof hydrogen / Gram atomic massof hydrogen
= 1000g/1g = 1000 molesof hydrogen
(ii)
At STP, 1 mole of any gas occupies 22.4 L.
So, at STP, 1000 moles of ``\ce{CO2}`` will occupy the same space as that of
hydrogen.
Atomic masses of C and O are 12 and 16, respectively.
Molar mass of ``CO_2`` = 12 + 32 = 44 g
1000 moles of ``CO_2 = 44 \times 1000 = 44000 g = 44 kg \ CO_2``
Thus, the cylinder can hold 44 kg ``CO_2``.
(iii) 1 mole of any gas = ``6.022 \times 10^{23}`` molecules
1000 moles of hydrogen = ``6.022 \times 10^{26}`` molecules = X
As the number of moles of hydrogen and CO2 are the same,
the number of molecules of CO2 = X = ``6.022 \times 10^{26}`` molecules of ``CO_2``.
(iv) Avogadro.s law states that under the same conditions of temperature
and pressure, equal volumes of different gases have the same number
of molecules.
- #5-c [2]Write a balanced chemical equation for the preparation of each of the following salts:
i. Copper carbonate
ii. Ammonium sulphate crystalsAns :
(i)
``\ce{CuO + CO2-> CuCO3}``
(ii) ``\ce{2NH3 + H2SO4 -> (NH4)2SO4}``
- #6Ans : 6.
- #6-a [4]Give a balanced chemical equation for each of the following:
i. Action of conc. Nitric acid on Sulphur.
ii. Catalytic oxidation of Ammonia.
iii. Laboratory preparation of Nitric acid.
iv. Reaction of Ammonia with Nitric acid.Ans :
(i)
``\ce{S + 6HNO3 -> H2SO4 + 2H2O + 6NO2}``
(ii)
``\ce{4NH3 + 5O2 ->[Pt.][800 ° C] 4NO + 6H2O + \ Heat \ }``
(iii)
``\ce{KNO3 + H2SO4 ->[<200 ° C] KHSO4 + HNO3 }``
(iv)
``\ce{NH3 + HNO3-> NH4NO3}``