ICSE-X-Chemistry
Previous Year Paper year:2017
- #1-c-vA solid formed by reaction of two gases, one of which is acidic and the other basic
in nature.Ans : Salt
- #1-d [5]Write a balanced chemical equation for each of the following:
- #1-d-iAction of cold and dilute Nitric acid on Copper.Ans : ``\ce{3Cu + 8HNO3 -> 3Cu(NO3)2 + 4H2O + 2NO(^)}``
- #1-d-iiReaction of Ammonia with heated copper oxide.Ans : ``\ce{3CuO + 3NH3 -> 3Cu + 3H2O + N2(^)}``
- #1-d-iiiPreparation of methane from iodomethane.Ans : ``\ce{CH3I + 2[H] -> CH4 + HI}``
- #1-d-ivAction of concentrated sulphuric acid on Sulphur.Ans : ``\ce{S + 2H2SO4 -> 3SO2 + 2H2O}``
- #1-d-vLaboratory preparation of ammonia from ammonium chloride.Ans : ``\ce{2NH4Cl + Ca(OH)2 -> CaCl2 + 2H2O + 2NH3(^)}``
- #1-e [5]State one relevant observation for each of the following reactions:
- #1-e-iAddition of ethyl alcohol to acetic acid in the presence of concentrated Sulphuric
acid.Ans : Sweet smelling ethyl acetate ester is
produced, and the process is known as esterification.
``\ce{C2H5OH + CH3COOH -> CH3COOC2H5 + H2O}``
- #1-e-iiAction of dilute Hydrochloric acid on iron (II) sulphide.Ans : hydrogen sulphide having rotten egg smell.
``\ce{FeS + 2HCl -> FeCl2 + H2S}``
- #1-e-iiiAction of Sodium hydroxide solution on ferrous sulphate solution.Ans : dirty green gelatinous ppt. of ferrous hydroxide is formed along with colourless
sodium sulphate.
``\ce{FeSO4 + 2NaOH -> Fe(OH)2. + Na2SO4}``
- #1-e-ivBurning of ammonia in air.Ans : it extinguishes a burning splint and does not burn in air.
- #1-e-vAction of concentrated Sulphuric acid on hydrated copper sulphate.Ans : Concentrated sulphuric acid removes water of crystallisation from
blue-coloured hydrated copper sulphate to form white anhydrous
copper sulphate.
``\ce{CuSO4.5H2O->CuSO4 +5H2O}``
Blue --- Dirty white
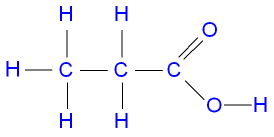
- #1-f [5](i) Draw the structural formula for each of the following:
1. 2, 3 . dimethyl butane
2. Diethyl ether
3. Propanoic acidAns :
(i)
1. 2, 3-dimethyl butane
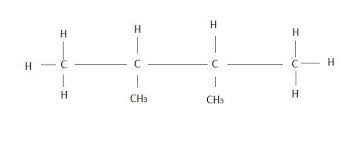
2. diethyl ether
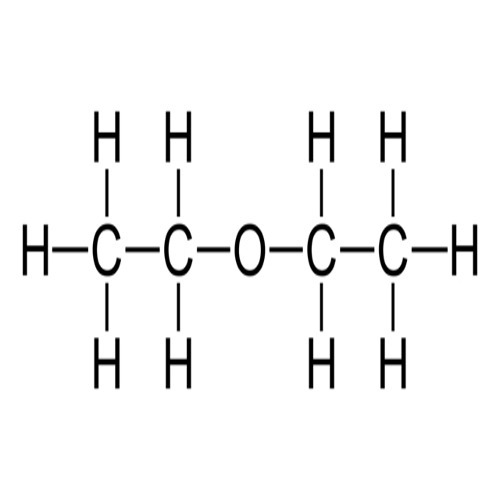
3. propanoic acid
- #1-f-iiSelect the process from the following which matches the given description.
(calcination, roasting, pulverisation, smelting)
1. Crushing of the ore into a fine powder.
2. Heating of the ore in the absence of air to a high temperature.Ans : 1. The process of crushing ores into a fine powder in big crushers and
ball mills is known as pulverisation.
2. Heating of the ore in the absence of air to a high temperature that
is high but insufficient to melt the ore is known as calcination.