ICSE-X-Chemistry
Previous Year Paper year:2019
- #1-i-iiiLi, K, Na, H (In the decreasing order of their ionization potential)
Ans : H > Li > Na > K
(Smaller the size greater is the ionization potential)
- #1-i-ivF, B, N, O (In the increasing order of electron affinity)
Ans : B < N < O < F
(Smaller the size more is the electron affinity)
- #1-i-vEthane, methane, ethene, ethyne. (In the increasing order of the molecular weight)
[H = 1, C = 12]
Ans :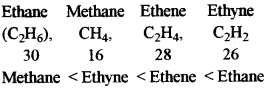
- #Section : II[40 Marks]
(Attempt any four questions from this Section)
- #2
- #2-aDraw the electron dot structure of :
[3]
- #2-a-iNitrogen molecule [N = 7]
Ans : Nitrogen molecule

Nitrogen atom shares three electrons forming a triple covalent bond.
- #2-a-iiSodium chloride [Na = 11, Cl = 17]
Ans : Sodium chloride
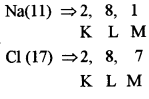
Sodium loses one electron to chlorine forming a positive ion and chlorine gains one electron forming a negative ion. These ions form an electrovalent bond and are held strongly by electrostatic forces of attraction.
- #2-a-iiiAmmonium ion [N = 7, H = 1]
Ans : Ammonium ion
N (7) →
H (1) → Hx
Formation of ammonia
Formation of proton
H - 1e- H+
Formation of ammonium ion
Ammonia donates its lone pair to proton forming ammonium ion.
- #2-bThe pH values of three solutions A, B and C are given in the table. Answer the following questions:
[3]

- #2-b-iWhich solution will have no effect on litmus solution?Ans : C (Because pH 7 is neutral).
- #2-b-iiWhich solution will liberate CO2when reacted with sodium carbonate?Ans : B (Because acids liberate CO2 gas when treated with carbonates and acids have pH less than 7)
- #2-b-iiiWhich solution will turn red litmus solution blue?Ans : A (Bases turn red litmus blue and they have pH more than 7)
- #2-cStudy the extract of the Periodic Table given below and answer the questions that follow. Give the alphabet corresponding to the element in question. DO NOT repeat an element.
[4]
- #2-c-iWhich element forms electrovalent compound with G?Ans :
