ICSE-X-Chemistry
Previous Year Paper year:2019
- #1-cWrite a balanced chemical equation for each of the following reactions:
[5]
- #1-c-iReduction of copper (II) oxide by hydrogen.
Ans : CuO+H2→ Cu+H2O
- #1-c-iiAction of dilute sulphuric acid on sodium hydroxide.
Ans : 2NaOH + H2S04 → Na2SO4 + 2H2O
- #1-c-iiiAction of dilute sulphuric acid on zinc sulphide.
Ans : ZnS + H2SO4 → ZnSO4 + H2S
- #1-c-ivAmmonium hydroxide is added to ferrous sulphate solution.
Ans : FeSO4 + 2NH40H → (NH4)SO4 + Fe(OH)2↓
- #1-c-vChlorine gas is reacted with ethene.
Ans :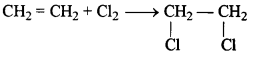
- #1-dState one observation for each of the following:
[5]
- #1-d-iConcentrated nitric acid is reacted with sulphur.
Ans :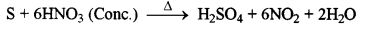
Dense brown fumes of nitrogen dioxide gas will be released
- #1-d-iiAmmonia gas is passed over heated copper (II) oxide.
Ans :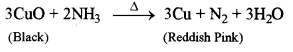
The black colour copper oxide will change to reddish pink copper.
- #1-d-iiiCopper sulphate solution is electrolysed using copper electrodes.
Ans : When copper sulphate solution is electrolysed using copper electrodes, reddish pink deposit of copper metal takes place on cathode.
- #1-d-ivA small piece of zinc is added to dilute hydrochloric acid.
Ans : Zn + 2HCl > ZnCl2 + H2↑
Zinc metal dissolves forming solution with the liberation of hydrogen gas which bums with blue flame and gets extinguished with pop sound.
- #1-d-vLead nitrate is heated strongly in a test tube.
Ans :
White powder of lead nitrate decompose to form yellow coloured lead oxide and liberating dense brown fumes of nitrogen dioxide gas.
- #1-e
- #1-e-iCalculate:
[5]
1. The number of moles in 12 g of oxygen gas. [O = 16]
2. The weight of 1022 atoms of carbon.
[C = 12, Avogadro’s No. = 6 × 1023]
Ans : 1. Oxygen gas (O2)
Molecular mass = 16 x 2 = 32 g
32 g of oxygen gas → 1 mole
1 g of oxygen gas →``\frac { 1 }{ 32 }`` mole
12 g of oxygen gas →``\frac { 1 }{ 32 }`` × 12mole = 0.375 mole
2. 6.022 x 1023 atoms of carbon weigh → 12 g
1 atom of carbon weighs →``\frac{12}{6 \times 10^{23}}``
1022 atoms of carbon will weigh →``\frac{12}{6 \times 10^{23}} \times 10^{22}=\frac{12}{60}=\frac{1}{5}`` = 0.2 g
- #1-e-iiMolecular formula of a compound is C6H18O3. Find its empirical formula.
Ans : Molecular formula = C6H18O3
Take the common multiple
Molecular formula = (C2H60)3
Molecular formula = (Empirical formula)„
Thus, empirical formula = C2H6O