CBSE-IX-Science
11: 2-Structure of the Atom
- #8Explain with examples
() Atomic number,
() Mass number,
() Isotopes and
iv) Isobars.
Give any two uses of isotopes.
Ans : i) Atomic number = It is the number of protons present inside nucleus of the atom.
It is represented as Z. For eg: for hydrogen Z= 1, because in hydrogen atom the number of protons is 1.
ii) Mass number = It is the total number of protons and neutrons present inside the nucleus of an atom and is represented by A = P + N mass of carbon is 12 u because it has 6 protons and 6 neutrons, 6 u + 6 u = 12 u.
iii) Isotopes = They are atoms of the same element and have same atomic number but different mass number/atomic mass. for example: carbon,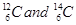
iv) Isobars = They are atoms of different elements having same mass number but different atomic number. for example, calcium, atomic number 20, and argon, atomic number 18. The number of electrons in these atoms is different, but the mass number of both these elements is 40. That is, the total number of neutrons is the same in the atoms of this pair of elements.
Two uses of isotopes are as follows:
() An isotope of uranium is used as a fuel in nuclear reactors.
() An isotope of cobalt is used in the treatment of cancer.
()
- #8-iAtomic number,Ans : An isotope of uranium is used as a fuel in nuclear reactors.
- #8-iiMass number,Ans : An isotope of cobalt is used in the treatment of cancer.
- #8-iiiIsotopes and
iv) Isobars.
Give any two uses of isotopes.