Loading…
CBSE-IX-Science
11: 2-Structure of the Atom
- #6Summarise the rules for writing of distribution of electrons in various shells for the first eighteen elements.Ans : The following rules are followed for writing the number of electrons in different energy levels or shells:
(i) The maximum number of electrons present in a shell is given by the formula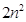 , where ‘n’ is the orbit number or energy level index, 1,2,3,... Hence the maximum number of electrons in different shells are as follows:
, where ‘n’ is the orbit number or energy level index, 1,2,3,... Hence the maximum number of electrons in different shells are as follows:
first orbit or K-shell will be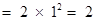 ,
,
second orbit or L-shell will be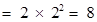 ,
,
third orbit or M-shell will be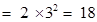 ,
,
fourth orbit or N-shell will be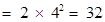 , and so on.
, and so on.
(ii) The maximum number of electrons that can be accommodated in the outermost orbit is 8.
(iii) Electrons are not accommodated in a given shell, unless the inner shells are filled. That is, the shells are filled in a step-wise manner.