CBSE-IX-Science
06: 1-Is Matter around Us Pure
- #Science Is Matter around Us Pure part 1
- #Section : I
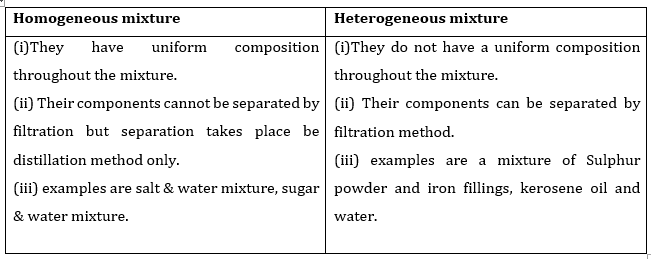
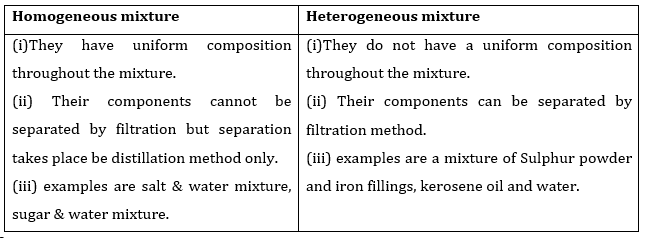
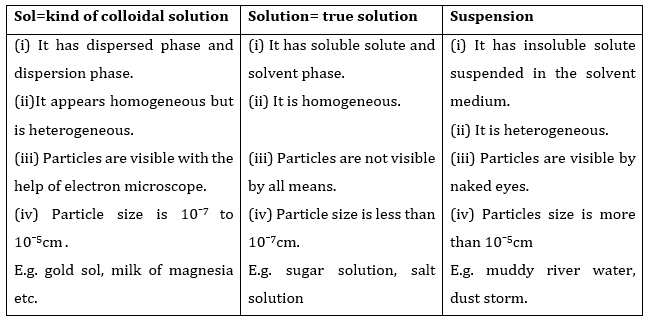
- Qstn #1What is meant by a substance?Ans : Substance can be defined as that kind of matter where constituent particles cannot be separated from each other by any physical process since they are all similar in chemical properties.
- #Section : II
- Qstn #3To make a saturated solution, 36 g of sodium chloride is dissolved in 100 g of water at 293 K. Find its concentration at this temperature.Ans : Mass of sodium chloride (solute) = 36 g
Mass of water (solvent) = 100 g
Mass of solution = 36 + 100 = 136 g
Therefore, concentration percentage = mass of solute/mass of solution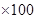
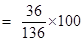 = 26.47 %
= 26.47 %
- #Section : III
- Qstn #1How will you separate a mixture containing kerosene and petrol (difference in their boiling points is more than
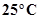 ), which are miscible with each other?Ans : We can separate a mixture containing kerosene and petrol by distillation technique since difference in their boiling points is more than
), which are miscible with each other?Ans : We can separate a mixture containing kerosene and petrol by distillation technique since difference in their boiling points is more than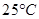 . So through distillation we can get them separated.
. So through distillation we can get them separated.
- Qstn #3What type of mixtures are separated by the technique of crystallisation?Ans : From impure samples of solids, pure solid crystals can be obtained by the method of crystallization for eg to obtain pure sugar from impure sample of the same.