NEET-XII-Physics
26: Laws of Thermodynamics
- #17Consider the cyclic process ABCA, shown in figure, performed on a sample of 2.0 mol of an ideal gas. A total of 1200 J of heat is withdrawn from the sample in the process. Find the work done by the gas during the part BC.
FigureAns : Given:
Number of moles of the gas, n = 2
∆Q = - 1200 J (Negative sign shows that heat is extracted out from the system)
∆U = 0 (During cyclic process)
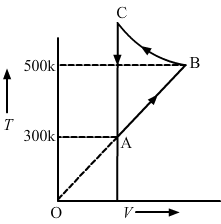
Using the first law of thermodynamics, we get
∆Q = ∆U + ∆W
⇒ -1200 = 0 + (WAB + WBC + WCA)
Since the change in volume of the system applies on line CA, work done during CA will be zero.
From the ideal gas equation, we get
PV = nRT
P∆V = nR∆T
W = P∆V = nR∆T
⇒ ∆Q = ∆U + ∆W
⇒ -1200 = nR∆T + WBC + 0
⇒ -1200 = 2 × 8.3 × 200 + WBC
WBC = - 400 × 8.3 - 1200
= - 4520 J
Page No 63: