NEET-XII-Physics
26: Laws of Thermodynamics
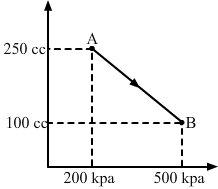
- #13A gas is taken along the path AB as shown in figure. If 70 cal of heat is extracted from the gas in the process, calculate the change in the internal energy of the system.
FigureAns : Given: 70 cal of heat is extracted from the system.
Here,
∆Q = `` -``70 cal = `` -``(70 × 4.2) J = `` -``294 J
From the first law of thermodynamics, we get
∆W = P`` ∆V``
If P is the average pressure between points A and B and `` ∆V`` is the change in volume of the system while going from point A to B, then
∆W = `` -```` \frac{1}{2}`` × (200 + 500) × 103 × (150 × 10-6)
∆W = `` -```` \frac{1}{2}`` × 700 × 150 × 10-3
∆W = `` -``525 × 10-1 = `` -``52.5 J
Here, negative sign is taken because the final volume is less than the initial volume.
∆U = ?
∆Q = ∆U + ∆W
∆Q = `` -``294 J
Here, negative sign indicates that heat is extracted out from the system.
⇒ - 294 = ∆U `` -`` 52.5
⇒ ∆U = - 294 + 52.5 = `` -`` 241.5 J
Page No 63: