NEET-XII-Physics
24: Kinetic Theory of Gases
- #6Figure shows graphs of pressure vs density for an ideal gas at two temperatures T1 and T2.
(a) T1 > T2
(b) T1 = T2
(c) T1 < T2
(d) Any of the three is possible.
Figure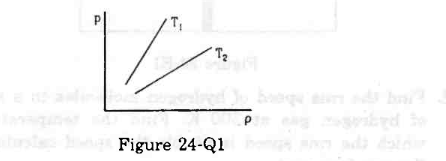 digAnsr: aAns : The straight line T1 has greater slope than T2. This means `` \frac{P}{\rho }`` ratio is greater for T1 than T2. Now, rms velocity of a gas is given by `` \sqrt{\frac{3P}{\rho }}``. This means rms velocity of gas with T1 molecules is greater than T2 molecules. Again, gas with higher temperature has higher rms velocity.
digAnsr: aAns : The straight line T1 has greater slope than T2. This means `` \frac{P}{\rho }`` ratio is greater for T1 than T2. Now, rms velocity of a gas is given by `` \sqrt{\frac{3P}{\rho }}``. This means rms velocity of gas with T1 molecules is greater than T2 molecules. Again, gas with higher temperature has higher rms velocity.
So, T1 > T2.
Thus,
(a) is the correct answer.