NEET-XII-Physics
24: Kinetic Theory of Gases
- #13The process on an ideal gas, shown in figure, is
(a) isothermal
(b) isobaric
(c) isochoric
(d) none of these.
Figure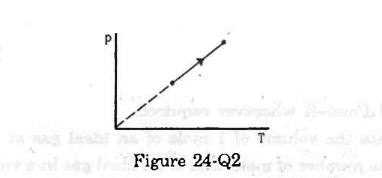 digAnsr: cAns : According to the graph, P is directly proportional to T.
digAnsr: cAns : According to the graph, P is directly proportional to T.
Applying the equation of state, we get
PV = nRT
`` \Rightarrow P=\frac{nR}{V}T``
`` ``
`` \,\mathrm{\,Given\,}:P\alpha T``
`` \,\mathrm{\,This\,}\,\mathrm{\,means\,}\frac{\,\mathrm{\,n\,}\,\mathrm{\,R\,}}{\,\mathrm{\,V\,}}\,\mathrm{\,is\,}\,\mathrm{\,a\,}\,\mathrm{\,constant\,}.\,\mathrm{\,So\,},\,\mathrm{\,V\,}\,\mathrm{\,is\,}\,\mathrm{\,also\,}\,\mathrm{\,a\,}\,\mathrm{\,constant\,}.``
Constant V implies the process is isochoric.
Thus,
(c) is the correct answer.